A Mechanism Linking Two Known Vulnerability Factors for Alcohol Abuse: Heightened Alcohol Stimulation and Low Striatal Dopamine D2 Receptors
- PMID: 31665630
- PMCID: PMC6880649
- DOI: 10.1016/j.celrep.2019.09.059
A Mechanism Linking Two Known Vulnerability Factors for Alcohol Abuse: Heightened Alcohol Stimulation and Low Striatal Dopamine D2 Receptors
Abstract
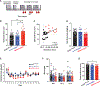
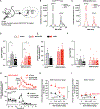
Alcohol produces both stimulant and sedative effects in humans and rodents. In humans, alcohol abuse disorder is associated with a higher stimulant and lower sedative responses to alcohol. Here, we show that this association is conserved in mice and demonstrate a causal link with another liability factor: low expression of striatal dopamine D2 receptors (D2Rs). Using transgenic mouse lines, we find that the selective loss of D2Rs on striatal medium spiny neurons enhances sensitivity to ethanol stimulation and generates resilience to ethanol sedation. These mice also display higher preference and escalation of ethanol drinking, which continues despite adverse outcomes. We find that striatal D1R activation is required for ethanol stimulation and that this signaling is enhanced in mice with low striatal D2Rs. These data demonstrate a link between two vulnerability factors for alcohol abuse and offer evidence for a mechanism in which low striatal D2Rs trigger D1R hypersensitivity, ultimately leading to compulsive-like drinking.
Keywords: alcohol use disorders; dopamine D1 receptors; dorsal medial striatum; ethanol; ethanol self-administration; loss of righting reflex; sedation; striatum.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





References
-
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, and Borrelli E (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

