Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial
- PMID: 31665733
- PMCID: PMC6889917
- DOI: 10.1159/000503712
Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial
Abstract
Background: Among people with diabetes, those with kidney disease have exceptionally high rates of cardiovascular (CV) morbidity and mortality and progression of their underlying kidney disease. Finerenone is a novel, nonsteroidal, selective mineralocorticoid receptor antagonist that has shown to reduce albuminuria in type 2 diabetes (T2D) patients with chronic kidney disease (CKD) while revealing only a low risk of hyperkalemia. However, the effect of finerenone on CV and renal outcomes has not yet been investigated in long-term trials.
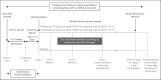
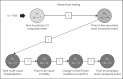
Patients and methods: The Finerenone in Reducing CV Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial aims to assess the efficacy and safety of finerenone compared to placebo at reducing clinically important CV and renal outcomes in T2D patients with CKD. FIGARO-DKD is a randomized, double-blind, placebo-controlled, parallel-group, event-driven trial running in 47 countries with an expected duration of approximately 6 years. FIGARO-DKD randomized 7,437 patients with an estimated glomerular filtration rate ≥25 mL/min/1.73 m2 and albuminuria (urinary albumin-to-creatinine ratio ≥30 to ≤5,000 mg/g). The study has at least 90% power to detect a 20% reduction in the risk of the primary outcome (overall two-sided significance level α = 0.05), the composite of time to first occurrence of CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure.
Conclusions: FIGARO-DKD will determine whether an optimally treated cohort of T2D patients with CKD at high risk of CV and renal events will experience cardiorenal benefits with the addition of finerenone to their treatment regimen.
Trial registration: EudraCT number: 2015-000950-39; ClinicalTrials.gov identifier: NCT02545049.
Keywords: Aldosterone; Clinical; Diabetes; Kidney; Mineralocorticoid; Outcomes.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
L.M.R. has served as an advisor/speaker for AstraZeneca, Bayer, Daiichi-Sankyo, Medtronic, Novartis, and Recordati. R.A. is a member of data safety monitoring committees for AstraZeneca and Ironwood Pharmaceuticals; a member of steering committees of randomized trials for Akebia, Bayer, Janssen, GlaxoSmithKline, Relypsa, and Sanofi and Genzyme US Companies; a member of adjudication committees for Bayer, Boehringer Ingelheim, and Janssen; and a member of a scientific advisory board or a consultant for Bird Rock Bio, Celgene, Daiichi Sankyo, Inc., Eli Lilly, Relypsa, Reata, Takeda Pharmaceuticals, USA, and ZS Pharma; he has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and has been an author for UpToDate; and he has received research grants from the U.S. Veterans Administration and the National Institutes of Health. S.D.A. has received research support from Abbott Vascular and Vifor International, and personal fees from Boehringer Ingelheim, Bayer, AstraZeneca, Novartis, Vifor International, Impulse Dynamics, Respicardia, and St. Jude Medical. G.L.B. reports research funding, paid to the University of Chicago, from Bayer, Janssen, and Vascular Dynamics; he has acted as a consultant for Merck, Vascular Dynamics, Relypsa, Boehringer Ingelheim, Sanofi, Pfizer, Novo Nordisk, Ionis, and AstraZeneca; he is an editor of the American Journal of Nephrology, Nephrology and Hypertension, and section editor of UpToDate; and he is associate editor of Diabetes Care and Hypertension Research. G.F. reports that he is a committee member of trials and registries sponsored by Bayer, Novartis, Servier, Vifor, Medtronic, and Boehringer Ingelheim. C.N., P.K., A.J., and N.M. are full-time employees of Bayer AG, Division Pharmaceuticals, Germany. B.P. reports consultant fees for Bayer, AstraZeneca, Sanofi, scPharmaceuticals, SQ Innovation, G3 Pharmaceuticals, Sarfez, Vifor, Cereno, Ardelyx, KBP Biosciences and Windtree Pharmaceuticals; he has stock options for KBP Biosciences, SQ Innovation, Sarfez, scPharmaceuticals, Cereno and G3 Pharmaceuticals, and Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent #9931412).
Figures
References
-
- Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States 1990-2010. N Engl J Med. 2014 Apr;370((16)):1514–23. - PubMed
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017 Jun;128:40–50. - PubMed
-
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease a 10-year population-based study of the effects of gender and age. Kidney Int. 2006 Jan;69((2)):375–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

