ApoE (Apolipoprotein E) in Brain Pericytes Regulates Endothelial Function in an Isoform-Dependent Manner by Modulating Basement Membrane Components
- PMID: 31665905
- PMCID: PMC7007705
- DOI: 10.1161/ATVBAHA.119.313169
ApoE (Apolipoprotein E) in Brain Pericytes Regulates Endothelial Function in an Isoform-Dependent Manner by Modulating Basement Membrane Components
Abstract
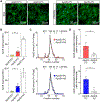
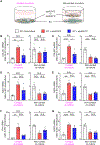
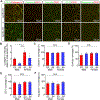
Objective: The ε4 allele of the APOE gene (APOE4) is the strongest genetic risk factor for Alzheimer disease when compared with the common ε3 allele. Although there has been significant progress in understanding how apoE4 (apolipoprotein E4) drives amyloid pathology, its effects on amyloid-independent pathways, in particular cerebrovascular integrity and function, are less clear. Approach and Results: Here, we show that brain pericytes, the mural cells of the capillary walls, differentially modulate endothelial cell phenotype in an apoE isoform-dependent manner. Extracellular matrix protein induction, tube-like structure formation, and barrier formation were lower with endothelial cells cocultured with pericytes isolated from apoE4-targeted replacement (TR) mice compared with those from apoE3-TR mice. Importantly, aged apoE4-targeted replacement mice had decreased extracellular matrix protein expression and increased plasma protein leakages compared with apoE3-TR mice.
Conclusions: ApoE4 impairs pericyte-mediated basement membrane formation, potentially contributing to the cerebrovascular effects of apoE4.
Keywords: Alzheimer disease; blood-brain barrier; collagen IV; extracellular matrix; pericytes; risk factors; tight junction.
Figures



Comment in
-
APOE at the BBB: Pericyte-Derived Apolipoprotein E4 Diminishes Endothelial Cell Barrier Function.Arterioscler Thromb Vasc Biol. 2020 Jan;40(1):14-16. doi: 10.1161/ATVBAHA.119.313627. Epub 2019 Dec 23. Arterioscler Thromb Vasc Biol. 2020. PMID: 31869265 Free PMC article. No abstract available.
References
-
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and alzheimer disease. A meta-analysis. Apoe and alzheimer disease meta analysis consortium. Jama. 1997;278:1349–1356 - PubMed
-
- Kamboh MI, Aston CE, Ferrell RE, Hamman RF. Impact of apolipoprotein e polymorphism in determining interindividual variation in total cholesterol and low density lipoprotein cholesterol in hispanics and non-hispanic whites. Atherosclerosis. 1993;98:201–211 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

