MCP1-CCR2 and neuroinflammation in the ALS motor cortex with TDP-43 pathology
- PMID: 31666087
- PMCID: PMC6822373
- DOI: 10.1186/s12974-019-1589-y
MCP1-CCR2 and neuroinflammation in the ALS motor cortex with TDP-43 pathology
Abstract
Background: The involvement of non-neuronal cells and the cells of innate immunity has been attributed to the initiation and progression of ALS. TDP-43 pathology is observed in a broad spectrum of ALS cases and is one of the most commonly shared pathologies. The potential involvement of the neuroimmune axis in the motor cortex of ALS patients with TDP-43 pathology needs to be revealed. This information is vital for building effective treatment strategies.
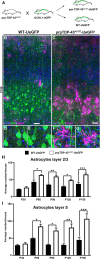
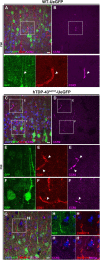
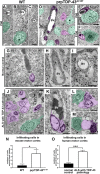
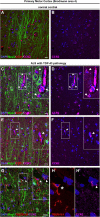
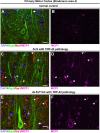
Methods: We investigated the presence of astrogliosis and microgliosis in the motor cortex of ALS patients with TDP-43 pathology. prpTDP-43A315T-UeGFP mice, corticospinal motor neuron (CSMN) reporter line with TDP-43 pathology, are utilized to reveal the timing and extent of neuroimmune interactions and the involvement of non-neuronal cells to neurodegeneration. Electron microscopy and immunolabeling techniques are used to mark and monitor cells of interest.
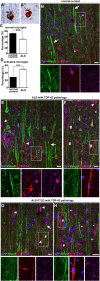
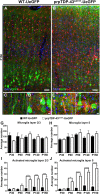
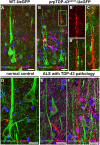
Results: We detected both activated astrocytes and microglia, especially rod-like microglia, in the motor cortex of patients and TDP-43 mouse model. Besides, CCR2+ TMEM119- infiltrating monocytes were detected as they penetrate the brain parenchyma. Interestingly, Betz cells, which normally do not express MCP1, were marked with high levels of MCP1 expression when diseased.
Conclusions: There is an early contribution of a neuroinflammatory response for upper motor neuron (UMN) degeneration with respect to TDP-43 pathology, and MCP1-CCR2 signaling is important for the recognition of diseased upper motor neurons by infiltrating monocytes. The findings are conserved among species and are observed in both ALS and ALS-FTLD patients.
Keywords: MCP1-CCR2 axis; Microglia; TDP-43; Upper motor neurons.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Evidence for an early innate immune response in the motor cortex of ALS.J Neuroinflammation. 2017 Jun 26;14(1):129. doi: 10.1186/s12974-017-0896-4. J Neuroinflammation. 2017. PMID: 28651542 Free PMC article.
-
Mitochondrial dysregulation occurs early in ALS motor cortex with TDP-43 pathology and suggests maintaining NAD+ balance as a therapeutic strategy.Sci Rep. 2022 Mar 11;12(1):4287. doi: 10.1038/s41598-022-08068-5. Sci Rep. 2022. PMID: 35277554 Free PMC article.
-
Mitochondria, ER, and nuclear membrane defects reveal early mechanisms for upper motor neuron vulnerability with respect to TDP-43 pathology.Acta Neuropathol. 2019 Jan;137(1):47-69. doi: 10.1007/s00401-018-1934-8. Epub 2018 Nov 19. Acta Neuropathol. 2019. PMID: 30450515 Free PMC article.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
-
The Molecular Link Between TDP-43, Endogenous Retroviruses and Inflammatory Neurodegeneration in Amyotrophic Lateral Sclerosis: a Potential Target for Triumeq, an Antiretroviral Therapy.Mol Neurobiol. 2023 Nov;60(11):6330-6345. doi: 10.1007/s12035-023-03472-y. Epub 2023 Jul 14. Mol Neurobiol. 2023. PMID: 37450244 Free PMC article. Review.
Cited by
-
Dysfunction of RNA/RNA-Binding Proteins in ALS Astrocytes and Microglia.Cells. 2021 Nov 3;10(11):3005. doi: 10.3390/cells10113005. Cells. 2021. PMID: 34831228 Free PMC article. Review.
-
Deciphering therapeutic options for neurodegenerative diseases: insights from SIRT1.J Mol Med (Berl). 2022 Apr;100(4):537-553. doi: 10.1007/s00109-022-02187-2. Epub 2022 Mar 11. J Mol Med (Berl). 2022. PMID: 35275221 Review.
-
CCR2 is localized in microglia and neurons, as well as infiltrating monocytes, in the lumbar spinal cord of ALS mice.Mol Brain. 2020 Apr 29;13(1):64. doi: 10.1186/s13041-020-00607-3. Mol Brain. 2020. PMID: 32349774 Free PMC article.
-
Amyotrophic lateral sclerosis, a neurodegenerative motor neuron disease with retinal involvement.Neural Regen Res. 2022 May;17(5):1011-1012. doi: 10.4103/1673-5374.324841. Neural Regen Res. 2022. PMID: 34558524 Free PMC article. No abstract available.
-
Cellular and molecular mechanisms of the blood-brain barrier dysfunction in neurodegenerative diseases.Fluids Barriers CNS. 2024 Jul 19;21(1):60. doi: 10.1186/s12987-024-00557-1. Fluids Barriers CNS. 2024. PMID: 39030617 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

