Identifying important barriers to recruitment of patients in randomised clinical studies using a questionnaire for study personnel
- PMID: 31666093
- PMCID: PMC6822437
- DOI: 10.1186/s13063-019-3737-1
Identifying important barriers to recruitment of patients in randomised clinical studies using a questionnaire for study personnel
Abstract
Background: Many randomised controlled trials (RCT) fail to meet their recruitment goals. Study personnel play a key role in recruitment. The aim of this study was to identify successful strategies that study personnel consider to be important in patient recruitment to RCT.
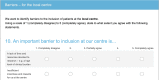
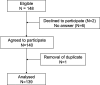
Methods: We constructed a questionnaire based on the literature, discussions with colleagues and our own experience as trialists. The survey was named "What is Important for Making a Study Successful questionnaire" (WIMSS-q). Our target group was the study personnel in the ongoing EFFECTS study. The questionnaire was sent out electronically to all physicians and nurses (n = 148). Success factors and barriers were divided according to patient, centre and study level, respectively.
Results: Responses were received from 94% of the study personnel (139/148). The five most important factors at centre level for enhancing recruitment were that the research question was important (97%), a simple procedure for providing information and gaining consent (92%), a highly engaged local principal investigator and research nurse (both 87%), and that study-related follow-ups are practically feasible and possible to coordinate with the clinical follow-up (87%). The most significant barrier at the local centre was lack of time and resources devoted to research (72%). Important patient-related barriers were fear of side effects (35%) and language problems (30%).
Conclusions: For recruitment in an RCT to be successful, the research question must be relevant, and the protocol must be simple and easy to implement in the daily routine.
Trial registration: The protocol for this study was registered at the Northern Ireland Hub for trials methodology research (SWAT ID 64 ). The EFFECTS study has EudraCT number 2011-006130-16 and was registered 17 February 2016 at ClinicalTrials.gov number NCT02683213 .
Keywords: Questionnaire; RCT; Randomised controlled trials; Recruitment; Survey.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007;11(48):iii. - PubMed
-
- Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs. 2010;19(1–2):227–233. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical