Tuberculosis Vaccine Development: Progress in Clinical Evaluation
- PMID: 31666281
- PMCID: PMC6822991
- DOI: 10.1128/CMR.00100-19
Tuberculosis Vaccine Development: Progress in Clinical Evaluation
Abstract
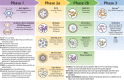
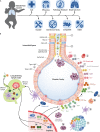
Tuberculosis (TB) is the leading killer among all infectious diseases worldwide despite extensive use of the Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccine. A safer and more effective vaccine than BCG is urgently required. More than a dozen TB vaccine candidates are under active evaluation in clinical trials aimed to prevent infection, disease, and recurrence. After decades of extensive research, renewed promise of an effective vaccine against this ancient airborne disease has recently emerged. In two innovative phase 2b vaccine clinical trials, one for the prevention of Mycobacterium tuberculosis infection in healthy adolescents and another for the prevention of TB disease in M. tuberculosis-infected adults, efficacy signals were observed. These breakthroughs, based on the greatly expanded knowledge of the M. tuberculosis infection spectrum, immunology of TB, and vaccine platforms, have reinvigorated the TB vaccine field. Here, we review our current understanding of natural immunity to TB, limitations in BCG immunity that are guiding vaccinologists to design novel TB vaccine candidates and concepts, and the desired attributes of a modern TB vaccine. We provide an overview of the progress of TB vaccine candidates in clinical evaluation, perspectives on the challenges faced by current vaccine concepts, and potential avenues to build on recent successes and accelerate the TB vaccine research-and-development trajectory.
Keywords: clinical trials; desired attributes; developmental trajectory; natural immunity; new TB vaccines; tuberculosis.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures



References
-
- World Health Organization. 2018. Global tuberculosis report 2018. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

