Pharmacologically Aware Phage Therapy: Pharmacodynamic and Pharmacokinetic Obstacles to Phage Antibacterial Action in Animal and Human Bodies
- PMID: 31666296
- PMCID: PMC6822990
- DOI: 10.1128/MMBR.00012-19
Pharmacologically Aware Phage Therapy: Pharmacodynamic and Pharmacokinetic Obstacles to Phage Antibacterial Action in Animal and Human Bodies
Abstract
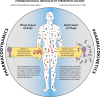
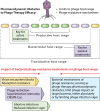
The use of viruses infecting bacteria (bacteriophages or phages) to treat bacterial infections has been ongoing clinically for approximately 100 years. Despite that long history, the growing international crisis of resistance to standard antibiotics, abundant anecdotal evidence of efficacy, and one successful modern clinical trial of efficacy, this phage therapy is not yet a mainstream approach in medicine. One explanation for why phage therapy has not been subject to more widespread implementation is that phage therapy research, both preclinical and clinical, can be insufficiently pharmacologically aware. Consequently, here we consider the pharmacological obstacles to phage therapy effectiveness, with phages in phage therapy explicitly being considered to serve as drug equivalents. The study of pharmacology has traditionally been differentiated into pharmacokinetic and pharmacodynamic aspects. We therefore separately consider the difficulties that phages as virions can have in traveling through body compartments toward reaching their target bacteria (pharmacokinetics) and the difficulties that phages can have in exerting antibacterial activity once they have reached those bacteria (pharmacodynamics). The latter difficulties, at least in part, are functions of phage host range and bacterial resistance to phages. Given the apparently low toxicity of phages and the minimal side effects of phage therapy as practiced, phage therapy should be successful so long as phages can reach the targeted bacteria in sufficiently high numbers, adsorb, and then kill those bacteria. Greater awareness of what obstacles to this success generally or specifically can exist, as documented in this review, should aid in the further development of phage therapy toward wider use.
Keywords: bacteriophage therapy; phage circulation; phage clearance; phage movement; phage resistance; spectrum of activity.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, Foster S, Gilmore BF, Hancock RE, Harper D, Henderson IR, Hilpert K, Jones BV, Kadioglu A, Knowles D, Olafsdottir S, Payne D, Projan S, Shaunak S, Silverman J, Thomas CM, Trust TJ, Warn P, Rex JH. 2016. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect Dis 16:239–251. doi:10.1016/S1473-3099(15)00466-1. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

