Incidence of necrotising enterocolitis before and after introducing routine prophylactic Lactobacillus and Bifidobacterium probiotics
- PMID: 31666311
- PMCID: PMC7363787
- DOI: 10.1136/archdischild-2019-317346
Incidence of necrotising enterocolitis before and after introducing routine prophylactic Lactobacillus and Bifidobacterium probiotics
Abstract
Objective: To compare rates of necrotising enterocolitis (NEC), late-onset sepsis, and mortality in 5-year epochs before and after implementation of routine daily multistrain probiotics administration in high-risk neonates.
Design: Single-centre retrospective observational study over the 10-year period from 1 January 2008 to 31 December 2017.
Setting: Level 3 neonatal intensive care unit (NICU) of the Norfolk and Norwich University Hospital, UK.
Patients: Preterm neonates at high risk of NEC: admitted to NICU within 3 days of birth at <32 weeks' gestation or at 32-36 weeks' gestation and of birth weight <1500 g.
Intervention: Prior to 1 January 2013 probiotics were not used. Thereafter, dual-species Lactobacillus acidophilus and Bifidobacterium bifidum combination probiotics were routinely administered daily to high-risk neonates; from April 2016 triple-species probiotics (L. acidophilus, B. bifidum, and B. longum subspecies infantis) were used.
Main outcome measures: Incidence of NEC (modified Bell's stage 2a or greater), late-onset sepsis, and mortality.
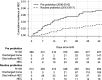
Results: Rates of NEC fell from 7.5% (35/469 neonates) in the pre-implementation epoch to 3.1% (16/513 neonates) in the routine probiotics epoch (adjusted sub-hazard ratio=0.44, 95% CI 0.23 to 0.85, p=0.014). The more than halving of NEC rates after probiotics introduction was independent of any measured covariates, including breast milk feeding rates. Cases of late-onset sepsis fell from 106/469 (22.6%) to 59/513 (11.5%) (p<0.0001), and there was no episode of sepsis due to Lactobacillus or Bifidobacterium. All-cause mortality also fell in the routine probiotics epoch, from 67/469 (14.3%) to 47/513 (9.2%), although this was not statistically significant after multivariable adjustment (adjusted sub-hazard ratio=0.74, 95% CI 0.49 to 1.12, p=0.155).
Conclusions: Administration of multispecies Lactobacillus and Bifidobacterium probiotics has been associated with a significantly decreased risk of NEC and late-onset sepsis in our neonatal unit, and no safety issues. Our data are consistent with routine use of Lactobacillus and Bifidobacterium combination probiotics having a beneficial effect on NEC prevention in very preterm neonates.
Keywords: Late-onset sepsis; microbiota; necrotizing enterocolitis; preterm; very low birth weight.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Grants and funding
- BB/J004529/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/00044409/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous
