Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease
- PMID: 31666338
- PMCID: PMC6901316
- DOI: 10.1074/jbc.REV119.007036
Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease
Abstract
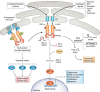
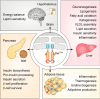
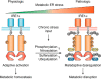
Inositol-requiring enzyme 1 (IRE1) is an endoplasmic reticulum (ER)-resident transmembrane protein that senses ER stress and is evolutionarily conserved from yeast to humans. IRE1 possesses both Ser/Thr protein kinase and endoribonuclease (RNase) activities within its cytoplasmic domain and is activated through autophosphorylation and dimerization/oligomerization. It mediates a critical arm of the unfolded protein response to manage ER stress provoked by lumenal overload of unfolded/misfolded proteins. Emerging lines of evidence have revealed that in mammals, IRE1α functions as a multifunctional signal transducer that responds to metabolic cues and nutrient stress conditions, exerting profound and broad effects on metabolic homeostasis. In this review, we cover recent advances in our understanding of how IRE1α integrates a variety of metabolic and stress signals and highlight its tissue-specific or context-dependent metabolic activities. We also discuss how dysregulation of this metabolic stress sensor during handling of excessive nutrients in cells contributes to the progression of obesity and metabolic disorders.
Keywords: ER-associated degradation; IRE1α; X-box–binding protein 1 (XBP1); endoplasmic reticulum stress (ER stress); endoplasmic reticulum to nucleus signaling 1 (Ern1); metabolic inflammation; nutrient sensing; regulated IRE1-dependent decay (RIDD); signal transduction; unfolded protein response (UPR).
© 2019 Huang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

