Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries
- PMID: 31666515
- PMCID: PMC6821766
- DOI: 10.1038/s41467-019-12857-4
Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries
Abstract
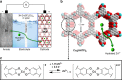
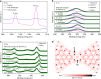
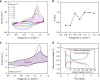
Currently, there is considerable interest in developing advanced rechargeable batteries that boast efficient distribution of electricity and economic feasibility for use in large-scale energy storage systems. Rechargeable aqueous zinc batteries are promising alternatives to lithium-ion batteries in terms of rate performance, cost, and safety. In this investigation, we employ Cu3(HHTP)2, a two-dimensional (2D) conductive metal-organic framework (MOF) with large one-dimensional channels, as a zinc battery cathode. Owing to its unique structure, hydrated Zn2+ ions which are inserted directly into the host structure, Cu3(HHTP)2, allow high diffusion rate and low interfacial resistance which enable the Cu3(HHTP)2 cathode to follow the intercalation pseudocapacitance mechanism. Cu3(HHTP)2 exhibits a high reversible capacity of 228 mAh g-1 at 50 mA g-1. At a high current density of 4000 mA g-1 (~18 C), 75.0% of the initial capacity is maintained after 500 cycles. These results provide key insights into high-performance, 2D conductive MOF designs for battery electrodes.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

