Cavitation-induced traumatic cerebral contusion and intracerebral hemorrhage in the rat brain by using an off-the-shelf clinical shockwave device
- PMID: 31666607
- PMCID: PMC6821893
- DOI: 10.1038/s41598-019-52117-5
Cavitation-induced traumatic cerebral contusion and intracerebral hemorrhage in the rat brain by using an off-the-shelf clinical shockwave device
Abstract
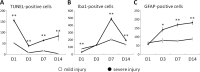
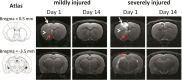
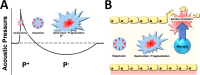
Traumatic cerebral contusion and intracerebral hemorrhages (ICH) commonly result from traumatic brain injury and are associated with high morbidity and mortality rates. Current animal models require craniotomy and provide less control over injury severity. This study proposes a highly reproducible and controllable traumatic contusion and ICH model using non-invasive extracorporeal shockwaves (ESWs). Rat heads were exposed to ESWs generated by an off-the-shelf clinical device plus intravenous injection of microbubbles to enhance the cavitation effect for non-invasive induction of injury. Results indicate that injury severity can be effectively adjusted by using different ESW parameters. Moreover, the location or depth of injury can be purposefully determined by changing the focus of the concave ESW probe. Traumatic contusion and ICH were confirmed by H&E staining. Interestingly, the numbers of TUNEL-positive cells (apoptotic cell death) peaked one day after ESW exposure, while Iba1-positive cells (reactive microglia) and GFAP-positive cells (astrogliosis) respectively peaked seven and fourteen days after exposure. Cytokine assay showed significantly increased expressions of IL-1β, IL-6, and TNF-α. The extent of brain edema was characterized with magnetic resonance imaging. Conclusively, the proposed non-invasive and highly reproducible preclinical model effectively simulates the mechanism of closed head injury and provides focused traumatic contusion and ICH.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Bullock MR, et al. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006;58:S25–46. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

