Inhaled nitric oxide as an adjunct to neonatal resuscitation in premature infants: a pilot, double blind, randomized controlled trial
- PMID: 31666688
- PMCID: PMC7223624
- DOI: 10.1038/s41390-019-0643-x
Inhaled nitric oxide as an adjunct to neonatal resuscitation in premature infants: a pilot, double blind, randomized controlled trial
Abstract
Background: Nitric oxide (NO) plays an important role in normal postnatal transition. Our aims were to determine whether adding inhaled NO (iNO) decreases supplemental oxygen exposure in preterm infants requiring positive pressure ventilation (PPV) during resuscitation and to study iNO effects on heart rate (HR), oxygen saturation (SpO2), and need for intubation during the first 20 min of life.
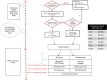
Methods: This was a pilot, double-blind, randomized, placebo-controlled trial. Infants 25 0/7-31 6/7 weeks' gestational age requiring PPV with supplemental oxygen during resuscitation were enrolled. PPV was initiated with either oxygen (FiO2-0.30) + iNO at 20 ppm (iNO group) or oxygen (FiO2-0.30) + nitrogen (placebo group). Oxygen was titrated targeting defined SpO2 per current guidelines. After 10 min, iNO/nitrogen was weaned stepwise per protocol and terminated at 17 min.
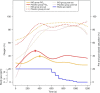
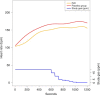
Results: Twenty-eight infants were studied (14 per group). The mean gestational age in both groups was similar. Cumulative FiO2 and rate of exposure to high FiO2 (>0.60) were significantly lower in the iNO group. There were no differences in HR, SpO2, and need for intubation.
Conclusions: Administration of iNO as an adjunct during neonatal resuscitation is feasible without side effects. It diminishes exposure to high levels of supplemental oxygen.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Nitric oxide and preterm resuscitation: some words of caution.Pediatr Res. 2020 Feb;87(3):438-440. doi: 10.1038/s41390-019-0649-4. Epub 2019 Oct 30. Pediatr Res. 2020. PMID: 31666690 No abstract available.

