Clinical parameters affecting multipotent adult progenitor cells in vitro
- PMID: 31667385
- PMCID: PMC6812213
- DOI: 10.1016/j.heliyon.2019.e02532
Clinical parameters affecting multipotent adult progenitor cells in vitro
Abstract
Background: Human multipotent adult progenitor cells (MAPC®) are an emerging therapy for traumatic brain injury (TBI); however, clinically translating a therapy involves overcoming many factors in vivo which are not present in pre-clinical testing. In this study we examined clinical parameters in vitro that may impact cell therapy efficacy.
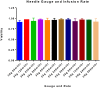
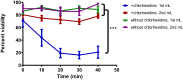
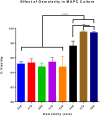
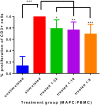
Methods: MAPC were infused through varying gauged needles and catheters with and without chlorhexidine, and their viability tested with trypan blue exclusion. MAPC were co-cultured with phenytoin and celecoxib at relevant clinical concentrations for 1 h and 24 h. Anti-inflammatory potency was tested using a stimulated rat splenocyte co-culture and ELISA for TNF-α production. MAPC were cultured under different osmolar concentrations and stained with propidium iodide for viability. Anti-inflammatory potency was tested by co-culture of MAPC with naïve lymphocytes activated by CD3/CD28 beads, and Click-iT® Plus EdU was used to quantify proliferation by flow cytometry.
Results: The mean viability of the MAPC infused via needles was 95 ± 1%; no difference was seen with varying flow rate, but viability was notably reduced by chlorhexidine. MAPC function was not impaired by co-culture with phenytoin, celecoxib, or combination with both. Co-culture with phenytoin showed a decrease in TNF-α production as compared to the MAPC control. MAPC cultured at varying osmolar concentrations all had viabilities greater than 90% with no statistical difference between them. Co-culture of MAPC with CD3/CD28 activated PBMCs showed a significant reduction in proliferation as measured by EdU uptake.
Discussion: Needle diameter, phenytoin, celecoxib, and a relevant range of osmolarities do not impair MAPC viability or anti-inflammatory potency in vitro.
Keywords: Cell biology; Cell therapy; Immunology; Inflammation; Medicine; Regenerative medicine; Stem cell research; Stem cells research; Translational medicine; Traumatic brain injury.
© 2019 The Author(s).
Figures


References
-
- Prevention, C. F. D. C. a. Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. 2019. https://stacks.cdc.gov/view/cdc/78062 - PMC - PubMed
-
- Prevention, C. F. D. C. a. Traumatic Brain Injury & Concussion: Get the Facts. 2019.
-
- Cox C.J., Juranek J., Bedi S. Clinical trials in traumatic brain injury: cellular therapy and outcome measures. Transfusion. 2019;59:858–868. - PubMed
-
- Walker P.A. Bone marrow–derived stromal cell therapy for traumatic brain injury is neuroprotective via stimulation of non-neurologic organ systems. Surgery. 2012;152:790–793. - PubMed
LinkOut - more resources
Full Text Sources

