Exocytosis of large-diameter lysosomes mediates interferon γ-induced relocation of MHC class II molecules toward the surface of astrocytes
- PMID: 31667557
- PMCID: PMC7391398
- DOI: 10.1007/s00018-019-03350-8
Exocytosis of large-diameter lysosomes mediates interferon γ-induced relocation of MHC class II molecules toward the surface of astrocytes
Abstract
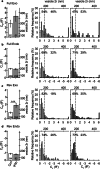
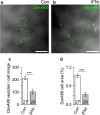
Astrocytes are the key homeostatic cells in the central nervous system; initiation of reactive astrogliosis contributes to neuroinflammation. Pro-inflammatory cytokine interferon γ (IFNγ) induces the expression of the major histocompatibility complex class II (MHCII) molecules, involved in antigen presentation in reactive astrocytes. The pathway for MHCII delivery to the astrocyte plasma membrane, where MHCII present antigens, is unknown. Rat astrocytes in culture and in organotypic slices were exposed to IFNγ to induce reactive astrogliosis. Astrocytes were probed with optophysiologic tools to investigate subcellular localization of immunolabeled MHCII, and with electrophysiology to characterize interactions of single vesicles with the plasmalemma. In culture and in organotypic slices, IFNγ augmented the astrocytic expression of MHCII, which prominently co-localized with lysosomal marker LAMP1-EGFP, modestly co-localized with Rab7, and did not co-localize with endosomal markers Rab4A, EEA1, and TPC1. MHCII lysosomal localization was corroborated by treatment with the lysosomolytic agent glycyl-L-phenylalanine-β-naphthylamide, which reduced the number of MHCII-positive vesicles. The surface presence of MHCII was revealed by immunolabeling of live non-permeabilized cells. In IFNγ-treated astrocytes, an increased fraction of large-diameter exocytotic vesicles (lysosome-like vesicles) with prolonged fusion pore dwell time and larger pore conductance was recorded, whereas the rate of endocytosis was decreased. Stimulation with ATP, which triggers cytosolic calcium signaling, increased the frequency of exocytotic events, whereas the frequency of full endocytosis was further reduced. In IFNγ-treated astrocytes, MHCII-linked antigen surface presentation is mediated by increased lysosomal exocytosis, whereas surface retention of antigens is prolonged by concomitant inhibition of endocytosis.
Keywords: Adaptive immunity; Astroglia; Fusion pore; Inflammatory cytokines; Lysosomes; Patch clamp.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

