Clinical outcomes and prognostic factors of stereotactic body radiation therapy combined with gemcitabine plus capecitabine for locally advanced unresectable pancreatic cancer
- PMID: 31667573
- PMCID: PMC11804411
- DOI: 10.1007/s00432-019-03066-z
Clinical outcomes and prognostic factors of stereotactic body radiation therapy combined with gemcitabine plus capecitabine for locally advanced unresectable pancreatic cancer
Abstract
Purpose: This study aimed to evaluate the clinical outcomes, toxicity, and prognostic factors of SBRT combined with gemcitabine plus capecitabine (GEM-CAP) in treating locally advanced pancreatic cancer (LAPC).
Methods: A total of 56 patients with LAPC treated with SBRT combined with GEM-CAP were reviewed from October 2010 to October 2016. The median total prescription dose at five fractions was 40 Gy (30-50 Gy). The patients were subjected to two cycles of GEM-CAP before SBRT. GEM-CAP chemotherapy was then offered for four cycles or until disease tolerance or progression. The primary endpoints included overall survival (OS) and progression-free survival (PFS).
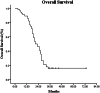
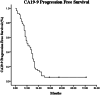
Results: The median OS and PFS from the date of diagnosis was 19 (95% CI 14.6-23.4) and 12 months (95% CI 8.34-15.66), respectively. The 1-year and 2-year survival rates were 82.1% and 35.7%, whereas the 1-year and 2-year PFS rates were 48.2% and 14.3%, respectively. The median carbohydrate antigen 19-9-determined PFS time was 11 months (95% CI 5.77-16.24). Multivariate analysis demonstrated that tumor diameter, lymph node metastasis, pre-treatment CA19-9 level, and post-treatment CA19-9 decline were independent prognostic factors (p < 0.05). Acute toxicity was minimal, with two cases (3.6%) experiencing grade 3 duodenal obstruction. No adverse events greater than grade 3 occurred. In late toxicity, three patients (5.4%) developed grade 3 gastrointestinal toxicity and two (3.6%) suffered from perforation caused by grade 4 radiation enteritis and intestinal fistula.
Conclusions: The combination of Cyberknife SBRT and GEM-CAP achieved excellent efficacy with acceptable toxicity for LAPC.
Keywords: CyberKnife; Locally advanced pancreatic cancer; Prognostic factors; Stereotactic body radiation therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37:4078–4101 - PubMed
-
- Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC (2009) Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 115:665–672 - PubMed
-
- Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L, Azzedine A, Hammel P, Butel J, Stremsdoerfer N, Maingon P, Bedenne L (2008) Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 19:1592–1599 - PubMed
-
- Cheng Z, Rosati LM, Chen L, Mian OY, Cao Y, Villafania M, Nakatsugawa M, Moore JA, Robertson SP, Jackson J, Hacker-Prietz A, He J, Wolfgang CL, Weiss MJ, Herman JM, Narang AK, McNutt TR (2018) Improving prediction of surgical resectability over current staging guidelines in patients with pancreatic cancer who receive stereotactic body radiation therapy. Adv Radiat Oncol 4:601–610 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

