Structure of the Decorated Ciliary Doublet Microtubule
- PMID: 31668805
- PMCID: PMC6936269
- DOI: 10.1016/j.cell.2019.09.030
Structure of the Decorated Ciliary Doublet Microtubule
Abstract
The axoneme of motile cilia is the largest macromolecular machine of eukaryotic cells. In humans, impaired axoneme function causes a range of ciliopathies. Axoneme assembly, structure, and motility require a radially arranged set of doublet microtubules, each decorated in repeating patterns with non-tubulin components. We use single-particle cryo-electron microscopy to visualize and build an atomic model of the repeating structure of a native axonemal doublet microtubule, which reveals the identities, positions, repeat lengths, and interactions of 38 associated proteins, including 33 microtubule inner proteins (MIPs). The structure demonstrates how these proteins establish the unique architecture of doublet microtubules, maintain coherent periodicities along the axoneme, and stabilize the microtubules against the repeated mechanical stress induced by ciliary motility. Our work elucidates the architectural principles that underpin the assembly of this large, repetitive eukaryotic structure and provides a molecular basis for understanding the etiology of human ciliopathies.
Keywords: axoneme; cilia; cryo-EM; doublet microtubule; tubulin.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare no competing interests.
Figures

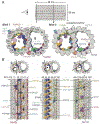





Comment in
-
Ciliary Doublet Microtubules at Near-Atomic Resolution.Cell. 2019 Oct 31;179(4):805-807. doi: 10.1016/j.cell.2019.10.013. Cell. 2019. PMID: 31675493
References
-
- Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, Forouhan M, Wilson R, Taylor-Cox T, Dewar A, Jackson C, et al. (2013). Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum. Mutat 34, 462–472. - PMC - PubMed
-
- Austin-Tse C, Halbritter J, Zariwala MA, Gilberti RM, Gee HY, Hellman N, Pathak N, Liu Y, Panizzi JR, Patel-King RS, et al. (2013). Zebrafish Ciliopathy Screen Plus Human Mutational Analysis Identifies C21orf59 and CCDC65 Defects as Causing Primary Ciliary Dyskinesia. Am. J. Hum. Genet 93, 672–686. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

