Direct current stimulation boosts hebbian plasticity in vitro
- PMID: 31668982
- PMCID: PMC6989352
- DOI: 10.1016/j.brs.2019.10.014
Direct current stimulation boosts hebbian plasticity in vitro
Abstract
Background: There is evidence that transcranial direct current stimulation (tDCS) can improve learning performance. Arguably, this effect is related to long term potentiation (LTP), but the precise biophysical mechanisms remain unknown.
Hypothesis: We propose that direct current stimulation (DCS) causes small changes in postsynaptic membrane potential during ongoing endogenous synaptic activity. The altered voltage dynamics in the postsynaptic neuron then modify synaptic strength via the machinery of endogenous voltage-dependent Hebbian plasticity. This hypothesis predicts that DCS should exhibit Hebbian properties, namely pathway specificity and associativity.
Methods: We studied the effects of DCS applied during the induction of LTP in the CA1 region of rat hippocampal slices and using a biophysical computational model.
Results: DCS enhanced LTP, but only at synapses that were undergoing plasticity, confirming that DCS respects Hebbian pathway specificity. When different synaptic pathways cooperated to produce LTP, DCS enhanced this cooperation, boosting Hebbian associativity. Further slice experiments and computer simulations support a model where polarization of postsynaptic pyramidal neurons drives these plasticity effects through endogenous Hebbian mechanisms. The model is able to reconcile several experimental results by capturing the complex interaction between the induced electric field, neuron morphology, and endogenous neural activity.
Conclusions: These results suggest that tDCS can enhance associative learning. We propose that clinical tDCS should be applied during tasks that induce Hebbian plasticity to harness this phenomenon, and that the effects should be task specific through their interaction with endogenous plasticity mechanisms. Models that incorporate brain state and plasticity mechanisms may help to improve prediction of tDCS outcomes.
Keywords: Hebbian; LTP; Synaptic plasticity; Transcranial direct current stimulation; Transcranial electrical stimulation; tDCS.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest LCP and MB have shares in Soterix Medical Inc and are listed as inventors in patents of the City University of New York related to high-definition tDCS.
Figures

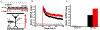
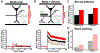


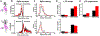


References
-
- Mancuso LE, Ilieva IP, Hamilton RH, Farah MJ. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-analytic Review. J Cogn Neurosci. 2016. April 7;28(8):1063–89. - PubMed
-
- Kekic M, Boysen E, Campbell IC, Schmidt U. A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. J Psychiatr Res. 2016. March 1;74:70–86. - PubMed
-
- Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt M-A. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimulat. 2016. July 1;9(4):501–17. - PubMed
-
- Tedesco Triccas L, Burridge JH, Hughes AM, Pickering RM, Desikan M, Rothwell JC, et al. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: A review and meta-analysis. Clin Neurophysiol. 2016. January 1;127(1):946–55. - PubMed
-
- Santarnecchi E, Brem A-K, Levenbaum E, Thompson T, Kadosh RC, Pascual-Leone A. Enhancing cognition using transcranial electrical stimulation. Curr Opin Behav Sci. 2015. August 1;4:171–8.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

