Development of protocols for the first serial block-face scanning electron microscopy (SBF SEM) studies of bone tissue
- PMID: 31669251
- PMCID: PMC6961117
- DOI: 10.1016/j.bone.2019.115107
Development of protocols for the first serial block-face scanning electron microscopy (SBF SEM) studies of bone tissue
Abstract
There is an unmet need for a high-resolution three-dimensional (3D) technique to simultaneously image osteocytes and the matrix in which these cells reside. In serial block-face scanning electron microscopy (SBF SEM), an ultramicrotome mounted within the vacuum chamber of a microscope repeatedly sections a resin-embedded block of tissue. Backscattered electron scans of the block face provide a stack of high-resolution two-dimensional images, which can be used to visualise and quantify cells and organelles in 3D. High-resolution 3D images of biological tissues from SBF SEM have been exploited considerably to date in the neuroscience field. However, non-brain samples, in particular hard biological tissues, have appeared more challenging to image by SBF SEM due to the difficulties of sectioning and rendering the samples conductive. We have developed and propose protocols for bone tissue preparation using SBF SEM, for imaging simultaneously soft and hard bone tissue components in 3D. We review the state of the art in high-resolution imaging of osteocytes, provide a historical perspective of SBF SEM, and we present first SBF SEM proof-of-concept studies for murine and human tissue. The application of SBF SEM to hard tissues will facilitate qualitative and quantitative 3D studies of tissue microstructure and ultrastructure in bone development, ageing and pathologies such as osteoporosis and osteoarthritis.
Keywords: 3D imaging; Bone; High resolution; Osteocyte; SBF SEM; Serial block-face scanning electron microscopy.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
At the time of this work SS was employed by Gatan UK, which manufactures the 3View® attachment for SBF SEM. He is now co-founder of ConnectomX, which manufactures the Katana Microtome. HG is employed by Diatome, a company manufacturing diamond knives for ultramicrotomy.
Figures


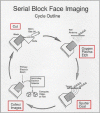



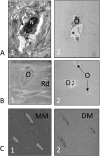

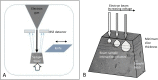
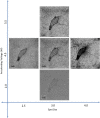

References
-
- Knothe Tate M.L., Adamson J.R., Tami A.E., Bauer T.W. The osteocyte. Int. J. Biochem. Cell Biol. 2004;36:1–8. - PubMed
-
- You L., Cowin S.C., Schaffler M.B., Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech. 2001;34:1375–1386. - PubMed
-
- Goggin P.M., Zygalakis K.C., Oreffo R.O., Schneider P. High-resolution 3D imaging of osteocytes and computational modelling in mechanobiology: insights on bone development, ageing, health and disease. Eur. Cell. Mater. 2016;31:264–295. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

