Differential metabolic and multi-tissue transcriptomic responses to fructose consumption among genetically diverse mice
- PMID: 31669422
- PMCID: PMC6993985
- DOI: 10.1016/j.bbadis.2019.165569
Differential metabolic and multi-tissue transcriptomic responses to fructose consumption among genetically diverse mice
Abstract
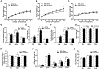
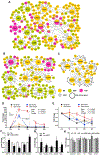
Understanding how individuals react differently to the same treatment is a major concern in precision medicine. Metabolic challenges such as the one posed by high fructose intake are important determinants of disease mechanisms. We embarked on studies to determine how fructose affects differential metabolic dysfunctions across genetically dissimilar mice, namely, C57BL/6 J (B6), DBA/2 J (DBA) and FVB/NJ (FVB), by integrating physiological and gene regulatory mechanisms. We report that fructose has strain-specific effects, involving tissue-specific gene regulatory cascades in hypothalamus, liver, and white adipose tissues. DBA mice showed the largest numbers of genes associated with adiposity, congruent with their highest susceptibility to adiposity gain and glucose intolerance across the three tissues. In contrast, B6 and FVB mainly exhibited cholesterol phenotypes, accompanying the largest number of adipose genes correlating with total cholesterol in B6, and liver genes correlating with LDL in FVB mice. Tissue-specific network modeling predicted strain-and tissue-specific regulators such as Fgf21 (DBA) and Lss (B6), which were subsequently validated in primary hepatocytes. Strain-specific fructose-responsive genes revealed susceptibility for human diseases such that genes in liver and adipose tissue in DBA showed strong enrichment for human type 2 diabetes and obesity traits. Liver and adipose genes in FVB were mostly related to lipid traits, and liver and adipose genes in B6 showed relevance to most cardiometabolic traits tested. Our results show that fructose induces gene regulatory pathways that are tissue specific and dependent on the genetic make-up, which may underlie interindividual variability in cardiometabolic responses to high fructose consumption.
Keywords: Fructose; Insulin resistance; Metabolic syndrome; Obesity; Personalized nutrition; Transcriptome.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
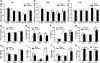
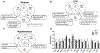
References
-
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT, The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men, JAMA, 288 (2002) 2709–2716. - PubMed
-
- Te Morenga L, Mallard S, Mann J, Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies, BMJ, 346 (2012) e7492. - PubMed
-
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ, Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans, J CLIN INVEST, 119 (2009) 1322–1334. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

