A characterization of Gaucher iPS-derived astrocytes: Potential implications for Parkinson's disease
- PMID: 31669751
- PMCID: PMC6980699
- DOI: 10.1016/j.nbd.2019.104647
A characterization of Gaucher iPS-derived astrocytes: Potential implications for Parkinson's disease
Abstract
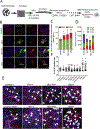
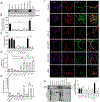
While astrocytes, the most abundant cells found in the brain, have many diverse functions, their role in the lysosomal storage disorder Gaucher disease (GD) has not been explored. GD, resulting from the inherited deficiency of the enzyme glucocerebrosidase and subsequent accumulation of glucosylceramide and its acylated derivative glucosylsphingosine, has both non-neuronopathic (GD1) and neuronopathic forms (GD2 and 3). Furthermore, mutations in GBA1, the gene mutated in GD, are an important risk factor for Parkinson's disease (PD). To elucidate the role of astrocytes in the disease pathogenesis, we generated iAstrocytes from induced pluripotent stem cells made from fibroblasts taken from controls and patients with GD1, with and without PD. We also made iAstrocytes from an infant with GD2, the most severe and progressive form, manifesting in infancy. Gaucher iAstrocytes appropriately showed deficient glucocerebrosidase activity and levels and substrate accumulation. These cells exhibited varying degrees of astrogliosis, Glial Fibrillary Acidic Protein (GFAP) up-regulation and cellular proliferation, depending on the level of residual glucocerebrosidase activity. Glutamte uptake assays demonstrated that the cells were functionally active, although the glutamine transporter EEAT2 was upregulated and EEAT1 downregulated in the GD2 samples. GD2 iAstrocytes were morphologically different, with severe cytoskeletal hypertrophy, overlapping of astrocyte processes, pronounced up-regulation of GFAP and S100β, and significant astrocyte proliferation, recapitulating the neuropathology observed in patients with GD2. Although astrocytes do not express α-synuclein, when the iAstrocytes were co-cultured with dopaminergic neurons generated from the same iPSC lines, excessive α-synuclein released from neurons was endocytosed by astrocytes, translocating into lysosomes. Levels of aggregated α-synuclein increased significantly when cells were treated with monomeric or fibrillar α-synuclein. GD1-PD and GD2 iAstrocytes also exhibited impaired Cathepsin D activity, leading to further α-synuclein accumulation. Cytokine and chemokine profiling of the iAstrocytes demonstrated an inflammatory response. Thus, in patients with GBA1-associated parkinsonism, astrocytes appear to play a role in α-synuclein accumulation and processing, contributing to neuroinflammation.
Keywords: Alpha-synuclein; Astrocytes; GBA1; Gaucher disease; Glucocerebrosidase; Induced pluriopotent stem cells; Parkinson's disease.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures






References
-
- Allen NJ, 2014. Synaptic plasticity: Astrocytes wrap it up. Curr Biol. 24, R697–9. - PubMed
-
- Braak H, et al. , 2007. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 114, 231–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

