Metformin protects against PM2.5-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase α2
- PMID: 31669973
- PMCID: PMC6838896
- DOI: 10.1016/j.redox.2019.101345
Metformin protects against PM2.5-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase α2
Abstract
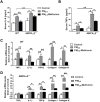
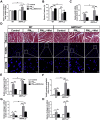
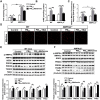
Fine particulate matter (PM2.5) airborne pollution increases the risk of respiratory and cardiovascular diseases. Although metformin is a well-known antidiabetic drug, it also confers protection against a series of diseases through the activation of AMP-activated protein kinase (AMPK). However, whether metformin affects PM2.5-induced adverse health effects has not been investigated. In this study, we exposed wild-type (WT) and AMPKα2-/- mice to PM2.5 every other day via intratracheal instillation for 4 weeks. After PM2.5 exposure, the AMPKα2-/- mice developed more severe lung injury and cardiac dysfunction than were developed in the WT mice; however the administration of metformin was effective in attenuating PM2.5-induced lung injury and cardiac dysfunction in both the WT and AMPKα2-/- mice. In the PM2.5-exposed mice, metformin treatment resulted in reduced systemic and pulmonary inflammation, preserved left ventricular ejection fraction, suppressed induction of pulmonary and myocardial fibrosis and oxidative stress, and increased levels of mitochondrial antioxidant enzymes. Moreover, pretreatment with metformin significantly attenuated PM2.5-induced cell death and oxidative stress in control and AMPKα2-depleted BEAS-2B and H9C2 cells, and was associated with preserved expression of mitochondrial antioxidant enzymes. These data support the notion that metformin protects against PM2.5-induced adverse health effects through a pathway that appears independent of AMPKα2. Our findings suggest that metformin may also be a novel drug for therapies that treat air pollution associated disease.
Keywords: AMPKα2; Cardiac dysfunction; Lung injury; Metformin; PM(2.5).
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Hopke P.K., Cohen D.D., Begum B.A., Biswas S.K., Ni B., Pandit G.G., Santoso M., Chung Y.S., Rahman S.A., Hamzah M.S., Davy P., Markwitz A., Waheed S., Siddique N., Santos F.L., Pabroa P.C., Seneviratne M.C., Wimolwattanapun W., Bunprapob S., Vuong T.B., Duy Hien P., Markowicz A. Urban air quality in the Asian region. Sci. Total Environ. 2008;404(1):103–112. - PubMed
-
- Deng X., Zhang F., Rui W., Long F., Wang L., Feng Z., Chen D., Ding W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. In Vitro : An International Journal Published in Association with BIBRA. 2013;27(6):1762–1770. - PubMed
-
- Tian L., Zhang W., Lin Z.Q., Zhang H.S., Xi Z.G., Chen J.H., Wang W. Impact of traffic emissions on local air quality and the potential toxicity of traffic-related particulates in Beijing, China. Biomed. Environ. Sci. : BES (Biomed. Environ. Sci.) 2012;25(6):663–671. - PubMed
-
- Li R., Kou X., Geng H., Xie J., Yang Z., Zhang Y., Cai Z., Dong C. Effect of ambient PM(2.5) on lung mitochondrial damage and fusion/fission gene expression in rats. Chem. Res. Toxicol. 2015;28(3):408–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

