Evidence for decreased Neurologic Pain Signature activation following thoracic spinal manipulation in healthy volunteers and participants with neck pain
- PMID: 31670070
- PMCID: PMC6831903
- DOI: 10.1016/j.nicl.2019.102042
Evidence for decreased Neurologic Pain Signature activation following thoracic spinal manipulation in healthy volunteers and participants with neck pain
Abstract
Background context: Spinal manipulation (SM) is a common treatment for neck and back pain, theorized to mechanically affect the spine leading to therapeutic mechanical changes. The link between specific mechanical effects and clinical improvement is not well supported. SM's therapeutic action may instead be partially mediated within the central nervous system.
Purpose: To introduce brain-based models of pain for spinal pain and manual therapy research, characterize the distributed central mechanisms of SM, and advance the preliminary validation of brain-based models as potential clinical biomarkers of pain.
Study design: Secondary analysis of two functional magnetic resonance imaging studies investigating the effect of thoracic SM on pain-related brain activity: A non-controlled, non-blinded study in healthy volunteers (Study 1, n = 10, 5 females, and mean age = 31.2 ± 10.0 years) and a randomized controlled study in participants with acute to subacute neck pain (Study 2, n = 24, 16 females, mean age = 38.0 ± 15.1 years).
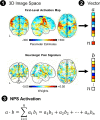
Methods: Functional magnetic resonance imaging was performed during noxious mechanical stimulation of the right index finger cuticle pre- and post-intervention. The effect of SM on pain-related activity was studied within brain regions defined by the Neurologic Pain Signature (NPS) that are predictive of physical pain.
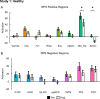
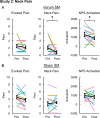
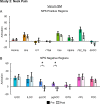
Results: In Study 1, evoked mechanical pain (p < 0.001) and NPS activation (p = 0.010) decreased following SM, and the changes in evoked pain and NPS activation were correlated (rRM2 = 0.418, p = 0.016). Activation within the NPS subregions of the dorsal anterior cingulate cortex (dACC, p = 0.012) and right secondary somatosensory cortex/operculum (rS2_Op, p = 0.045) also decreased following SM, and evoked pain was correlated with dACC activity (rRM2 = 0.477, p = 0.019). In Study 2, neck pain (p = 0.046) and NPS (p = 0.033) activation decreased following verum but not sham SM. Associations between evoked pain, neck pain, and NPS activation, were not significant and less clear, possibly due to inadequate power, methodological limitations, or other confounding factors.
Conclusions: The findings provide preliminary evidence that SM may alter the processing of pain-related brain activity within specific pain-related brain regions and support the use of brain-based models as clinical biomarkers of pain.
Keywords: Functional magnetic resonance imaging; Humans; Neck pain; Neuroimaging; Pain; Pain measurement; Randomized controlled trial; Spinal manipulation.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Drs. Weber, Wager, Mackey and Elliott report grants from the National Institutes of Health during the conduct of this study. Dr. Wager reports a Small Business Innovation Research grant with WaviMed. Dr. Wager is also on the scientific advisory board of Curable Health, Inc., has consulted for GSK and Cognifisense, has performed contract work for PainQX, and has been issued two patents: US 2016/0,054,409 fMRI-based Neurologic Signature of Physical Pain (PCT/US14/3353) and US 2018/0,055,407 Neurophysiological Signatures for Fibromyalgia (CU4199B-PPA1). Dr. Elliott is an advisory member for the board of directors of the Journal of Orthopaedic and Sports Physical Therapy, an editorial board member for the Journal of Orthopaedic and Sports Physical Therapy, an editorial board member for Musculoskeletal Science and Practice, and an advisory board member for Spine. Drs. Liu and Sparks declare no competing interests.
Figures



References
-
- Andersson J.L.R., Jenkinson M., Smith S. 2010. Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2.
-
- Apkarian A.V., Krauss B.R., Fredrickson B.E., Szeverenyi N.M. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci. Lett. 2001;299(1–2):57–60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

