Cycles, waves, and pulses: Retinoic acid and the organization of spermatogenesis
- PMID: 31670467
- PMCID: PMC7496180
- DOI: 10.1111/andr.12722
Cycles, waves, and pulses: Retinoic acid and the organization of spermatogenesis
Abstract
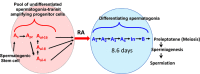
Background: Spermatogenesis in mammals is organized in a manner that maximizes sperm production. The central aspect of this organization is the cycle of the seminiferous epithelium that is characterized by an asynchronous repeating series of germ cell associations. These cell associations are the result of a fixed point of entry into the cycle at regular short time intervals and the longer time required for cells to fully differentiate and exit the cycle.
Objective: This review will examine the current information on the action and metabolism of retinoic acid in the testis, the interaction of retinoic acid (RA) with the cycle and the spermatogenic wave, and the mechanisms that can lead to synchronous spermatogenesis. Finally, the unique applications of synchronous spermatogenesis to the study of the cycle and the mass isolation of specific germ cell populations are described.
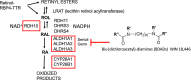
Materials and methods: Retinoic acid metabolism and spermatogonial differentiation have been examined by gene deletions, immunocytochemistry, chemical inhibitors, and mass spectrometry.
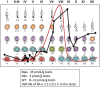
Results, discussion, and conclusion: Both the Sertoli cells and the germ cells have the capacity to synthesize retinoic acid from retinol and in the mouse the entry into the cycle of the seminiferous epithelium, and the subsequent conversion of undifferentiated spermatogonia into differentiating spermatogonia is governed by a peak of RA synthesis occurring at stages VIII-IX of the cycle. Normal asynchronous spermatogenesis can be modified by altering RA levels, and as a result the entire testis will consist of a few closely related stages of the cycle.
Keywords: cycle; retinoic acid; synchronization; wave.
© 2019 The Authors. Andrology published by Wiley Periodicals, Inc. on behalf of American Society of Andrology and European Academy of Andrology.
Figures

References
-
- Regaud C. Etudes sur la structure des tubes seminiferes et sur la spermatogenese chez les Mammiferes. Arch Anat Micr. 1901;4(101–156):231‐280.
-
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548‐573. - PubMed
-
- Franca LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371‐1377. - PubMed
-
- Perey B, Clermont Y, Leblond CP. The wave of the seminiferous epithelium in the rat. Am J Anat. 1961;108:47‐78.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

