Mitochondrial dysfunctions in leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL)
- PMID: 31671122
- PMCID: PMC6822708
- DOI: 10.1371/journal.pone.0224173
Mitochondrial dysfunctions in leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL)
Abstract
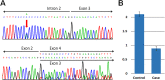
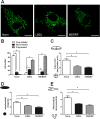
Several inherited human diseases have been linked to mitochondrial aminoacyl-tRNA synthetases (mtARSs). Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL) is a leukodystrophy caused by mutations in the DARS2 gene which encodes mitochondrial aspartyl-tRNA synthetase. As mitochondrial ARSs are key components of the mitochondrial translation apparatus, we investigated the effects of DARS2 mutations on mitochondrial functions and mitochondrial morphology in an LBSL patient. In fibroblasts from the patient with LBSL, biosynthesis of respiratory chain complex proteins encoded by mitochondrial DNA was decreased, while those encoded by nuclear DNA were not. Cellular oxygen consumption rates and respiratory control ratio were decreased in the LBSL patient; in addition, fragmentation of mitochondria was increased, while their tubular elongation and interconnectivity were decreased. Taken together, these findings suggest that DARS2 mutations impair translations of mitochondrial DNA-encoded respiratory chain complex proteins, consequently causing dysfunction of cellular respiration and impediment of mitochondrial dynamics, which highlights the role of mtARSs in the maintenance of normal mitochondrial bioenergetics and dynamics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

