Leptin induces cell migration and invasion in a FAK-Src-dependent manner in breast cancer cells
- PMID: 31671408
- PMCID: PMC6893313
- DOI: 10.1530/EC-19-0442
Leptin induces cell migration and invasion in a FAK-Src-dependent manner in breast cancer cells
Abstract
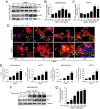
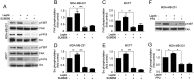
Breast cancer is the most common invasive neoplasia, and the second leading cause of the cancer deaths in women worldwide. Mammary tumorigenesis is severely linked to obesity, one potential connection is leptin. Leptin is a hormone secreted by adipocytes, which contributes to the progression of breast cancer. Cell migration, metalloproteases secretion, and invasion are cellular processes associated with various stages of metastasis. These processes are regulated by the kinases FAK and Src. In this study, we utilized the breast cancer cell lines MCF7 and MDA-MB-231 to determine the effect of leptin on FAK and Src kinases activation, cell migration, metalloprotease secretion, and invasion. We found that leptin activates FAK and Src and induces the localization of FAK to the focal adhesions. Interestingly, leptin promotes the activation of FAK through a Src- and STAT3-dependent canonical pathway. Specific inhibitors of FAK, Src and STAT3 showed that the effect exerted by leptin in cell migration in breast cancer cells is dependent on these proteins. Moreover, we established that leptin promotes the secretion of the extracellular matrix remodelers, MMP-2 and MMP-9 and invasion in a FAK and Src-dependent manner. Our findings strongly suggest that leptin promotes the development of a more aggressive invasive phenotype in mammary cancer cells.
Keywords: FAK; Src; breast cancer; cell migration; invasion; leptin; metalloproteases.
Figures






Similar articles
-
Leptin Promotes Expression of EMT-Related Transcription Factors and Invasion in a Src and FAK-Dependent Pathway in MCF10A Mammary Epithelial Cells.Cells. 2019 Sep 24;8(10):1133. doi: 10.3390/cells8101133. Cells. 2019. PMID: 31554180 Free PMC article.
-
The Leptin induced Hic-5 expression and actin puncta formation by the FAK/Src-dependent pathway in MCF10A mammary epithelial cells.Biomedica. 2019 Sep 1;39(3):547-560. doi: 10.7705/biomedica.4313. Biomedica. 2019. PMID: 31584768 Free PMC article. English, Spanish.
-
Leptin induces partial epithelial-mesenchymal transition in a FAK-ERK dependent pathway in MCF10A mammary non-tumorigenic cells.Int J Clin Exp Pathol. 2017 Oct 1;10(10):10334-10342. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966368 Free PMC article.
-
Interleukin-1β activates focal adhesion kinase and Src to induce matrix metalloproteinase-9 production and invasion of MCF-7 breast cancer cells.Oncol Lett. 2017 Feb;13(2):955-960. doi: 10.3892/ol.2016.5521. Epub 2016 Dec 20. Oncol Lett. 2017. PMID: 28356984 Free PMC article.
-
Role of Src/FAK in migration and invasion mediated by extracellular vesicles from MDA-MB-231 cells stimulated with linoleic acid.Med Oncol. 2021 Mar 16;38(4):40. doi: 10.1007/s12032-021-01485-y. Med Oncol. 2021. PMID: 33728516
Cited by
-
Hyperleptinemia in obese state renders luminal breast cancers refractory to tamoxifen by coordinating a crosstalk between Med1, miR205 and ErbB.NPJ Breast Cancer. 2021 Aug 13;7(1):105. doi: 10.1038/s41523-021-00314-9. NPJ Breast Cancer. 2021. PMID: 34389732 Free PMC article.
-
Adipocyte-derived IL6 and triple-negative breast cancer cell-derived CXCL1 co-activate STAT3/NF-κB pathway to mediate the crosstalk between adipocytes and triple-negative breast cancer cells.Cell Death Discov. 2025 Aug 21;11(1):395. doi: 10.1038/s41420-025-02713-4. Cell Death Discov. 2025. PMID: 40841364 Free PMC article.
-
Galectin-3 Mediates Thrombin-Induced Vascular Smooth Muscle Cell Migration.Front Cardiovasc Med. 2021 Oct 20;8:686200. doi: 10.3389/fcvm.2021.686200. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34746246 Free PMC article.
-
Regulation of cellular and molecular markers of epithelial-mesenchymal transition by Brazilin in breast cancer cells.PeerJ. 2024 May 9;12:e17360. doi: 10.7717/peerj.17360. eCollection 2024. PeerJ. 2024. PMID: 38737746 Free PMC article.
-
A luciferase fragment complementation assay to detect focal adhesion kinase (FAK) signaling events.Heliyon. 2023 Apr 5;9(4):e15282. doi: 10.1016/j.heliyon.2023.e15282. eCollection 2023 Apr. Heliyon. 2023. PMID: 37089315 Free PMC article.
References
-
- Iskander K, Farhour R, Ficek M, Ray A. Obesity-related complications: few biochemical phenomena with reference to tumorigenesis. Malaysian Journal of Pathology 2013. 1–15. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

