Implementation of Case-Based Surveillance and Real-time Polymerase Chain Reaction to Monitor Bacterial Meningitis Pathogens in Chad
- PMID: 31671450
- PMCID: PMC6822964
- DOI: 10.1093/infdis/jiz366
Implementation of Case-Based Surveillance and Real-time Polymerase Chain Reaction to Monitor Bacterial Meningitis Pathogens in Chad
Abstract
Background: Meningococcal serogroup A conjugate vaccine (MACV) was introduced in Chad during 2011-2012. Meningitis surveillance has been conducted nationwide since 2003, with case-based surveillance (CBS) in select districts from 2012. In 2016, the MenAfriNet consortium supported Chad to implement CBS in 4 additional districts and real-time polymerase chain reaction (rt-PCR) at the national reference laboratory (NRL) to improve pathogen detection. We describe analysis of bacterial meningitis cases during 3 periods: pre-MACV (2010-2012), pre-MenAfriNet (2013-2015), and post-MenAfriNet (2016-2018).
Methods: National surveillance targeted meningitis cases caused by Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae. Cerebrospinal fluid specimens, inoculated trans-isolate media, and/or isolates from suspected meningitis cases were tested via culture, latex, and/or rt-PCR; confirmed bacterial meningitis was defined by a positive result on any test. We calculated proportion of suspected cases with a specimen received by period, and proportion of specimens with a bacterial meningitis pathogen identified, by period, pathogen, and test.
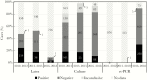
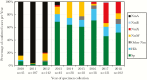
Results: The NRL received specimens for 6.8% (876/12813), 46.4% (316/681), and 79.1% (787/995) of suspected meningitis cases in 2010-2012, 2013-2015, and 2016-2018, respectively, with a bacterial meningitis pathogen detected in 33.6% (294/876), 27.8% (88/316), and 33.2% (261/787) of tested specimens. The number of N. meningitidis serogroup A (NmA) among confirmed bacterial meningitis cases decreased from 254 (86.4%) during 2010-2012 to 2 (2.3%) during 2013-2015, with zero NmA cases detected after 2014. In contrast, proportional and absolute increases were seen between 2010-2012, 2013-2015, and 2016-2018 in cases caused by S. pneumoniae (5.1% [15/294], 65.9% [58/88], and 52.1% [136/261]), NmX (0.7% [2/294], 1.1% [1/88], and 22.2% [58/261]), and Hib (0.3% [1/294], 11.4% [10/88], and 14.9% [39/261]). Of specimens received at the NRL, proportions tested during the 3 periods were 47.7% (418), 53.2% (168), and 9.0% (71) by latex; 81.4% (713), 98.4% (311), and 93.9% (739) by culture; and 0.0% (0), 0.0% (0), and 90.5% (712) by rt-PCR, respectively. During the post-MenAfriNet period (2016-2018), 86.1% (678) of confirmed cases were tested by both culture and rt-PCR, with 12.5% (85) and 32.4% (220) positive by culture and rt-PCR, respectively.
Conclusions: CBS implementation was associated with increased specimen referral. Increased detection of non-NmA cases could reflect changes in incidence or increased sensitivity of case detection with rt-PCR. Continued surveillance with the use of rt-PCR to monitor changing epidemiology could inform the development of effective vaccination strategies.
Keywords: Chad; MenAfriNet; bacterial meningitis; culture; latex; rt-PCR; sub-Saharan Africa.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- Greenwood B. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg 1999; 93:341–53. - PubMed
-
- Trotter CL, Lingani C, Fernandez K, et al. . Impact of MenAfriVac in nine countries of the African meningitis belt, 2010-15: an analysis of surveillance data. Lancet Infect Dis 2017; 17:867–72. - PubMed
-
- Garcia V, Morel B, Wadack MA, et al. . Outbreak of meningitis in the province of Logone occidental (Chad): descriptive study using health ministry data from 1998 to 2001. Bull Soc Pathol Exot 2004; 97:183–88. - PubMed

