The Potential Utility of Aripiprazole Augmentation for Major Depressive Disorder with Mixed Features Specifier: A Retrospective Study
- PMID: 31671486
- PMCID: PMC6852679
- DOI: 10.9758/cpn.2019.17.4.495
The Potential Utility of Aripiprazole Augmentation for Major Depressive Disorder with Mixed Features Specifier: A Retrospective Study
Abstract
Objective: The present study aimed to observe potential benefit of aripiprazole augmentation in the treatment of major depressive disorder with mixed specifier (MDDM) in naturalistic treatment setting.
Methods: Data were collected from MDDM patients using a retrospective chart review for 8 weeks (week -8 and week 0) in routine practice. All patients were on current antidepressants upon starting of aripiprazole. Patients were treated without restriction of doses of aripiprazole. The primary endpoint was the mean change of Montgomery-Åsberg Depression Rating Scale (MADRS) total scores along with various secondary endpoint measures.
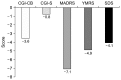
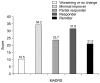
Results: In total 38 patients were analyzed. The changes of MADRS, Clinical Global Impression (CGI)-severity, Young Mania Rating Scale, Sheehan Disability Scale, and CGI-clinical benefit total scores from baseline to the endpoint were -7.1, -0.8, -4.9, -4.1, and -3.6, respectively (all p < 0.0001). At the endpoint, the responder and remitter rates by MADRS score criteria were approximately 32% and 21%, respectively.
Conclusion: The present findings have clearly shown the effectiveness and tolerability of aripiprazole augmentation for MDDM patients in routine practice. The present study warrants subsequent, adequately-powered, well-controlled studies for generalizability near future.
Keywords: Aripiprazole; Depressive disorder; Effectiveness; Mixed specifier; Tolerability..
Conflict of interest statement
This study was funded by a grant from Korea Otsuka Pharmaceutical. The funding source has not involved in any activities such as study protocol development, study design, data collection, data interpretation, and paper writing.
Figures


Similar articles
-
The Potential Role of Aripiprazole Augmentation for Major Depressive Disorder with Anxious Distress in Naturalistic Treatment Setting.Clin Psychopharmacol Neurosci. 2024 May 31;22(2):370-375. doi: 10.9758/cpn.23.1106. Epub 2024 Feb 4. Clin Psychopharmacol Neurosci. 2024. PMID: 38627084 Free PMC article.
-
Aripiprazole augmentation versus antidepressant switching for patients with major depressive disorder: A 6-week, randomized, rater-blinded, prospective study.J Psychiatr Res. 2015 Jul-Aug;66-67:84-94. doi: 10.1016/j.jpsychires.2015.04.020. Epub 2015 May 5. J Psychiatr Res. 2015. PMID: 26013203 Clinical Trial.
-
Additional Reduction of Residual Symptoms with Aripiprazole Augmentation in the Patients with Partially Remitted Major Depressive Disorder.Clin Psychopharmacol Neurosci. 2021 May 31;19(2):243-253. doi: 10.9758/cpn.2021.19.2.243. Clin Psychopharmacol Neurosci. 2021. PMID: 33888653 Free PMC article.
-
Aripiprazole as augmentation therapy in bipolar patients with current minor or subsyndromal mood symptoms.Int J Bipolar Disord. 2013 Apr 17;1:4. doi: 10.1186/2194-7511-1-4. eCollection 2013. Int J Bipolar Disord. 2013. PMID: 25505671 Free PMC article.
-
Aripiprazole: in major depressive disorder.CNS Drugs. 2008;22(10):807-13. doi: 10.2165/00023210-200822100-00002. CNS Drugs. 2008. PMID: 18788833 Review.
Cited by
-
Korean Medication Algorithm for Depressive Disorder 2021, Fourth Revision: An Executive Summary.Clin Psychopharmacol Neurosci. 2021 Nov 30;19(4):751-772. doi: 10.9758/cpn.2021.19.4.751. Clin Psychopharmacol Neurosci. 2021. PMID: 34690130 Free PMC article.
-
Effectiveness and Tolerability of Korean Red Ginseng Augmentation in Major Depressive Disorder Patients with Difficult-to-treat in Routine Practice.Clin Psychopharmacol Neurosci. 2020 Nov 30;18(4):621-626. doi: 10.9758/cpn.2020.18.4.621. Clin Psychopharmacol Neurosci. 2020. PMID: 33124595 Free PMC article.
-
Augmentative Pharmacological Strategies in Treatment-Resistant Major Depression: A Comprehensive Review.Int J Mol Sci. 2021 Dec 2;22(23):13070. doi: 10.3390/ijms222313070. Int J Mol Sci. 2021. PMID: 34884874 Free PMC article. Review.
-
The Potential Role of Aripiprazole Augmentation for Major Depressive Disorder with Anxious Distress in Naturalistic Treatment Setting.Clin Psychopharmacol Neurosci. 2024 May 31;22(2):370-375. doi: 10.9758/cpn.23.1106. Epub 2024 Feb 4. Clin Psychopharmacol Neurosci. 2024. PMID: 38627084 Free PMC article.
-
The Korean Medication Algorithm Project for Depressive Disorder (KMAP-DD): Changes in Preferred Treatment Strategies and Medications over 20 Years and Five Editions.J Clin Med. 2023 Feb 1;12(3):1146. doi: 10.3390/jcm12031146. J Clin Med. 2023. PMID: 36769798 Free PMC article.
References
-
- Han C, Wang SM, Bahk WM, Lee SJ, Patkar AA, Masand PS, et al. A pharmacogenomic-based antidepressant treatment for patients with major depressive disorder: results from an 8-week, randomized, single-blinded clinical trial. Clin Psychopharmacol Neurosci. 2018;16:469–480. doi: 10.9758/cpn.2018.16.4.469. - DOI - PMC - PubMed
-
- Dold M, Bartova L, Souery D, Mendlewicz J, Serretti A, Porcelli S, et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders - results from a European multi-center study. J Psychiatr Res. 2017;91:1–13. doi: 10.1016/j.jpsychires.2017.02.020. - DOI - PubMed
-
- McIntyre RS, Soczynska JK, Cha DS, Woldeyohannes HO, Dale RS, Alsuwaidan MT, et al. The prevalence and illness characteristics of DSM-5-defined “mixed feature specifier” in adults with major depressive disorder and bipolar disorder: results from the International Mood Disorders Collaborative Project. J Affect Disord. 2015;172:259–264. doi: 10.1016/j.jad.2014.09.026. - DOI - PubMed
LinkOut - more resources
Full Text Sources

