Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling
- PMID: 31671686
- PMCID: PMC6920939
- DOI: 10.3390/pharmaceutics11110564
Customized Novel Design of 3D Printed Pregabalin Tablets for Intra-Gastric Floating and Controlled Release Using Fused Deposition Modeling
Abstract
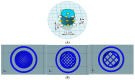
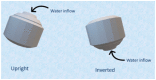
Three-dimensional (3D) printing has been recently employed in the design and formulation of various dosage forms with the aim of on-demand manufacturing and personalized medicine. In this study, we formulated a floating sustained release system using fused deposition modeling (FDM). Filaments were prepared using hypromellose acetate succinate (HPMCAS), polyethylene glycol (PEG 400) and pregabalin as the active ingredient. Cylindrical tablets with infill percentages of 25%, 50% and 75% were designed and printed with the FDM printer. An optimized formulation (F6) was designed with a closed bottom layer and a partially opened top layer. Filaments and tablets were characterized by means of fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), and thermogravimetric analysis (TGA). The results show that the processing condition did not have a significant effect on the stability of the drug and the crystallinity of the drug remained even after printing. A dissolution study revealed that drug release is faster in an open system with low infill percentage compared to closed systems and open systems with a high infill ratio. The optimized formulation (F6) with partially opened top layer showed zero-order drug release. The results show that FDM printing is suitable for the formulation of floating dosage form with the desired drug release profile.
Keywords: 3D printing; FMD; controlled release; gastric floating; pregabalin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Kodama H. Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev. Sci. Instrum. 1981;52:1770–1773. doi: 10.1063/1.1136492. - DOI
-
- Attaran M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017;60:677–688. doi: 10.1016/j.bushor.2017.05.011. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

