The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors
- PMID: 31672794
- PMCID: PMC6895474
- DOI: 10.2215/CJN.00990119
The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors
Abstract
Background and objectives: Immune checkpoint inhibitor use in oncology is increasing rapidly. We sought to determine the frequency, severity, cause, and predictors of AKI in a real-world population receiving checkpoint inhibitors.
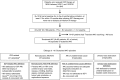
Design, setting, participants, & measurements: We included all patients who received checkpoint inhibitor therapy from May 2011 to December 2016 at Massachusetts General Hospital. Baseline serum creatinine, averaged 6 months before checkpoint inhibitor start date, was compared with all subsequent creatinine values within 12 months of starting therapy. AKI was defined by Kidney Disease: Improving Global Outcomes criteria for fold changes in creatinine from baseline. Sustained AKI events lasted at least 3 days and was our primary outcome. The cause of sustained AKI was determined by chart review. Cumulative incidence and subdistribution hazard models were used to assess the relationship between baseline demographics, comorbidities, and medications, and sustained AKI and potential checkpoint inhibitor-related AKI.
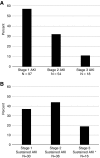
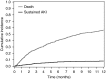
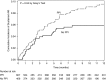
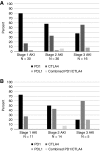
Results: We included 1016 patients in the analysis. Average age was 63 (SD 13) years, 61% were men, and 91% were white. Mean baseline creatinine was 0.9 mg/dl (SD 0.4 mg/dl), and 169 (17%) had CKD (eGFR<60 ml/min per 1.73 m2) at baseline. A total of 169 patients (17%) experienced AKI, defined by an increase in creatinine at least 1.5 times the baseline within 12 months; 82 patients (8%) experienced sustained AKI and 30 patients (3%) had potential checkpoint inhibitor-related AKI. The first episode of sustained AKI occurred, on average, 106 days (SD 85) after checkpoint inhibitor initiation. Sixteen (2%) patients experienced stage 3 sustained AKI and four patients required dialysis. Proton pump inhibitor use at baseline was associated with sustained AKI.
Conclusions: AKI is common in patients receiving checkpoint inhibitor therapy. The causes of sustained AKI in this population are heterogenous and merit thorough evaluation. The role of PPI and other nephritis-inducing drugs in the development of sustained AKI needs to be better defined.
Keywords: Massachusetts; acute interstitial nephritis; acute kidney injury; acute renal failure; checkpoint inhibitors; chronic renal insufficiency; comorbidity; creatinine; general hospitals; glomerular filtration rate; humans; immune related adverse events; incidence; kidney function tests; male; nephritis; nephrology; onconephrology; proportional hazards models; proton pump inhibitors; renal dialysis; risk factors.
Copyright © 2019 by the American Society of Nephrology.
Figures

Comment in
-
Acute Kidney Injury with Immune Checkpoint Inhibitors: A Push beyond Case Reports.Clin J Am Soc Nephrol. 2019 Dec 6;14(12):1679-1681. doi: 10.2215/CJN.12621019. Epub 2019 Oct 31. Clin J Am Soc Nephrol. 2019. PMID: 31672795 Free PMC article. No abstract available.
References
-
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723, 2010 - PMC - PubMed
-
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34, 2015 - PMC - PubMed
-
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR: Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627–1639, 2015 - PMC - PubMed
-
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators: Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803–1813, 2015 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

