Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium
- PMID: 31672839
- PMCID: PMC7295005
- DOI: 10.1136/gutjnl-2019-319352
Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium
Erratum in
-
Correction: Management of patients with increased risk for familial pancreatic cancer: updated recommendations for the international cancer of the pancreas screening (CAPS) Consortium.Gut. 2020 Jun;69(6):e3. doi: 10.1136/gutjnl-2019-319352corr1. Gut. 2020. PMID: 32381557 No abstract available.
Abstract
Background and aim: The International Cancer of the Pancreas Screening Consortium met in 2018 to update its consensus recommendations for the management of individuals with increased risk of pancreatic cancer based on family history or germline mutation status (high-risk individuals).
Methods: A modified Delphi approach was employed to reach consensus among a multidisciplinary group of experts who voted on consensus statements. Consensus was considered reached if ≥75% agreed or disagreed.
Results: Consensus was reached on 55 statements. The main goals of surveillance (to identify high-grade dysplastic precursor lesions and T1N0M0 pancreatic cancer) remained unchanged. Experts agreed that for those with familial risk, surveillance should start no earlier than age 50 or 10 years earlier than the youngest relative with pancreatic cancer, but were split on whether to start at age 50 or 55. Germline ATM mutation carriers with one affected first-degree relative are now considered eligible for surveillance. Experts agreed that preferred surveillance tests are endoscopic ultrasound and MRI/magnetic retrograde cholangiopancreatography, but no consensus was reached on how to alternate these modalities. Annual surveillance is recommended in the absence of concerning lesions. Main areas of disagreement included if and how surveillance should be performed for hereditary pancreatitis, and the management of indeterminate lesions.
Conclusions: Pancreatic surveillance is recommended for selected high-risk individuals to detect early pancreatic cancer and its high-grade precursors, but should be performed in a research setting by multidisciplinary teams in centres with appropriate expertise. Until more evidence supporting these recommendations is available, the benefits, risks and costs of surveillance of pancreatic surveillance need additional evaluation.
Keywords: early detection; familial pancreatic cancer; genetic predisposition; pancreatic ductal adenocarcinoma; surveillance.
© Author(s) (or their employer(s)) 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors disclose the following: JEvH received research funding from Abbott and Cook Medical; she is a consultant to Boston Scientific, Cook Medical, and Medtronics. DLC is a consultant to Tramedico. MB received research funding from Boston Scientific, Cook Medical, Pentax Medical, 3M; he is a consultant to Boston Scientific, Cook Medical, Pentax Medical, Mylan, MediRisk, and Medicom. PF is a consultant to Olympus, Cook Medical, Ethicon Endosurgery and received research funding from Boston Scientific. RB has received research funding from Immunovia and Freenome. MIC received research funding from Pentax C2 Cryoballoon and Endogastric Solutions. DS received research funding from Immunovia, Sanofi and Tempus; she is on the Scientific Advisory Board for Nybo Therapeutics, Interpace and Tyme.
Figures

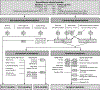
References
-
- Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012;16:771–83. - PubMed
-
- Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006;4:766–81. - PubMed
-
- Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009;58:1410–8. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
