Targeting Novel Sodium Iodide Symporter Interactors ADP-Ribosylation Factor 4 and Valosin-Containing Protein Enhances Radioiodine Uptake
- PMID: 31672844
- PMCID: PMC7470018
- DOI: 10.1158/0008-5472.CAN-19-1957
Targeting Novel Sodium Iodide Symporter Interactors ADP-Ribosylation Factor 4 and Valosin-Containing Protein Enhances Radioiodine Uptake
Abstract
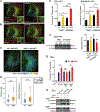
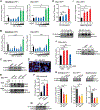
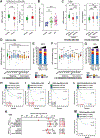
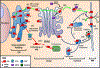
The sodium iodide symporter (NIS) is required for iodide uptake, which facilitates thyroid hormone biosynthesis. NIS has been exploited for over 75 years in ablative radioiodine (RAI) treatment of thyroid cancer, where its ability to transport radioisotopes depends on its localization to the plasma membrane. The advent of NIS-based in vivo imaging and theranostic strategies in other malignancies and disease modalities has recently increased the clinical importance of NIS. However, NIS trafficking remains ill-defined. Here, we used tandem mass spectrometry followed by coimmunoprecipitation and proximity ligation assays to identify and validate two key nodes-ADP-ribosylation factor 4 (ARF4) and valosin-containing protein (VCP)-controlling NIS trafficking. Using cell-surface biotinylation assays and highly inclined and laminated optical sheet microscopy, we demonstrated that ARF4 enhanced NIS vesicular trafficking from the Golgi to the plasma membrane, whereas VCP-a principal component of endoplasmic reticulum (ER)-associated degradation-governed NIS proteolysis. Gene expression analysis indicated VCP expression was particularly induced in aggressive thyroid cancers and in patients who had poorer outcomes following RAI treatment. Two repurposed FDA-approved VCP inhibitors abrogated VCP-mediated repression of NIS function, resulting in significantly increased NIS at the cell-surface and markedly increased RAI uptake in mouse and human thyroid models. Collectively, these discoveries delineate NIS trafficking and highlight the new possibility of systemically enhancing RAI therapy in patients using FDA-approved drugs. SIGNIFICANCE: These findings show that ARF4 and VCP are involved in NIS trafficking to the plasma membrane and highlight the possible therapeutic role of VCP inhibitors in enhancing radioiodine effectiveness in radioiodine-refractory thyroid cancer.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures



References
-
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1–133. - PMC - PubMed
-
- Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol 2014;2:356–8. - PubMed
-
- Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol 2014;10:830–42. - PubMed
-
- La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015; 136:2187–95. - PubMed
-
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature 1996;379:458–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

