Resolving the topological enigma in Ca2+ signaling by cyclic ADP-ribose and NAADP
- PMID: 31672920
- PMCID: PMC6937575
- DOI: 10.1074/jbc.REV119.009635
Resolving the topological enigma in Ca2+ signaling by cyclic ADP-ribose and NAADP
Abstract
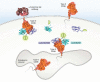
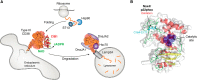
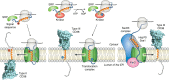
Cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) are two structurally distinct messengers that mobilize the endoplasmic and endolysosomal Ca2+ stores, respectively. Both are synthesized by the CD38 molecule (CD38), which has long been thought to be a type II membrane protein whose catalytic domain, intriguingly, faces to the outside of the cell. Accordingly, for more than 20 years, it has remained unresolved how CD38 can use cytosolic substrates such as NAD and NADP to produce messengers that target intracellular Ca2+ stores. The discovery of type III CD38, whose catalytic domain faces the cytosol, has now begun to clarify this topological conundrum. This article reviews the ideas and clues leading to the discovery of the type III CD38; highlights an innovative approach for uncovering its natural existence; and discusses the regulators of its activity, folding, and degradation. We also review the compartmentalization of cADPR and NAADP biogenesis. We further discuss the possible mechanisms that promote type III CD38 expression and appraise a proposal of a Ca2+-signaling mechanism based on substrate limitation and product translocation. The surprising finding of another enzyme that produces cADPR and NAADP, sterile α and TIR motif-containing 1 (SARM1), is described. SARM1 regulates axonal degeneration and has no sequence similarity with CD38 but can catalyze the same set of multireactions and has the same cytosolic orientation as the type III CD38. The intriguing finding that SARM1 is activated by nicotinamide mononucleotide to produce cADPR and NAADP suggests that it may function as a regulated Ca2+-signaling enzyme like CD38.
Keywords: CD38; SARM1; calcium intracellular release; calcium signaling; cyclic ADP ribose (cADPR); nicotinic acid adenine dinucleotide phosphate (NAADP); protein topology; signal transduction.
© 2019 Lee and Zhao.
Figures


References
-
- Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., and Clapper D. L. (1989) Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 264, 1608–1615 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

