Progenitor cell combination normalizes retinal vascular development in the oxygen-induced retinopathy (OIR) model
- PMID: 31672944
- PMCID: PMC6948778
- DOI: 10.1172/jci.insight.129224
Progenitor cell combination normalizes retinal vascular development in the oxygen-induced retinopathy (OIR) model
Abstract
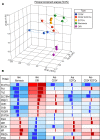
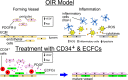
Retinopathy of prematurity (ROP) is a disorder of the developing retina of preterm infants. ROP can lead to blindness because of abnormal angiogenesis that is the result of suspended vascular development and vaso-obliteration leading to severe retinal stress and hypoxia. We tested the hypothesis that the use of the human progenitor cell combination, bone marrow-derived CD34+ cells and vascular wall-derived endothelial colony-forming cells (ECFCs), would synergistically protect the developing retinal vasculature in a mouse model of ROP, called oxygen-induced retinopathy (OIR). CD34+ cells alone, ECFCs alone, or the combination thereof were injected intravitreally at either P5 or P12 and pups were euthanized at P17. Retinas from OIR mice injected with ECFCs or the combined treatment revealed formation of the deep vascular plexus (DVP) while still in hyperoxia, with normal-appearing connections between the superficial vascular plexus (SVP) and the DVP. In addition, the combination of cells completely prevented aberrant retinal neovascularization and was more effective anatomically and functionally at rescuing the ischemia phenotype than either cell type alone. We show that the beneficial effects of the cell combination are the result of their ability to orchestrate an acceleration of vascular development and more rapid ensheathment of pericytes on the developing vessels. Lastly, our proteomic and transcriptomic data sets reveal pathways altered by the dual cell therapy, including many involved in neuroretinal maintenance, and principal component analysis (PCA) showed that cell therapy restored OIR retinas to a state that was closely associated with age-matched normal retinas. Together, these data herein support the use of dual cell therapy as a promising preventive treatment for the development of ROP in premature infants.
Keywords: Adult stem cells; Angiogenesis; Stem cells; hypoxia.
Conflict of interest statement
Figures


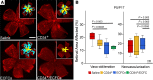
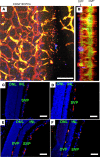
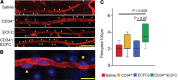
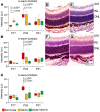


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

