A novel nucleoside rescue metabolic pathway may be responsible for therapeutic effect of orally administered cordycepin
- PMID: 31673018
- PMCID: PMC6823370
- DOI: 10.1038/s41598-019-52254-x
A novel nucleoside rescue metabolic pathway may be responsible for therapeutic effect of orally administered cordycepin
Abstract
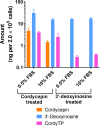
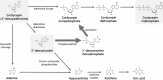
Although adenosine and its analogues have been assessed in the past as potential drug candidates due to the important role of adenosine in physiology, only little is known about their absorption following oral administration. In this work, we have studied the oral absorption and disposition pathways of cordycepin, an adenosine analogue. In vitro biopharmaceutical properties and in vivo oral absorption and disposition of cordycepin were assessed in rats. Despite the fact that numerous studies showed efficacy following oral dosing of cordycepin, we found that intact cordycepin was not absorbed following oral administration to rats. However, 3'-deoxyinosine, a metabolite of cordycepin previously considered to be inactive, was absorbed into the systemic blood circulation. Further investigation was performed to study the conversion of 3'-deoxyinosine to cordycepin 5'-triphosphate in vitro using macrophage-like RAW264.7 cells. It demonstrated that cordycepin 5'-triphosphate, the active metabolite of cordycepin, can be formed not only from cordycepin, but also from 3'-deoxyinosine. The novel nucleoside rescue metabolic pathway proposed in this study could be responsible for therapeutic effects of adenosine and other analogues of adenosine following oral administration. These findings may have importance in understanding the physiology and pathophysiology associated with adenosine, as well as drug discovery and development utilising adenosine analogues.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Carver JD, Walker WA. The Role of Nucleotides in Human-Nutrition. J Nutr Biochem. 1995;6(2):58–72. doi: 10.1016/0955-2863(94)00019-I. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

