Optimal Inhibition of Choroidal Neovascularization by scAAV2 with VMD2 Promoter-driven Active Rap1a in the RPE
- PMID: 31673119
- PMCID: PMC6823539
- DOI: 10.1038/s41598-019-52163-z
Optimal Inhibition of Choroidal Neovascularization by scAAV2 with VMD2 Promoter-driven Active Rap1a in the RPE
Abstract
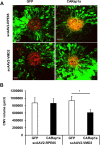
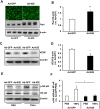
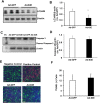
Age-related macular degeneration (AMD) is a multifactorial chronic disease that requires long term treatment. Gene therapy is being considered as a promising tool to treat AMD. We found that increased activation of Rap1a in the retinal pigment epithelium (RPE) reduces oxidative signaling to maintain barrier integrity of the RPE and resist neural sensory retinal angiogenesis from choroidal endothelial cell invasion. To optimally deliver constitutively active Rap1a (CARap1a) into the RPE of wild type mice, self-complementary AAV2 (scAAV2) vectors driven by two different promoters, RPE65 or VMD2, were generated and tested for optimal active Rap1a expression and inhibition of choroidal neovascularization (CNV) induced by laser injury. scAAV2-VMD2, but not scAAV2-RPE65, specifically and efficiently transduced the RPE to increase active Rap1a protein in the RPE. Mice with increased Rap1a from the scAAV2-VMD2-CARap1a had a significant reduction in CNV compared to controls. Increased active Rap1a in the RPE in vivo or in vitro inhibited inflammatory and angiogenic signaling determined by decreased activation of NF-κB and expression of VEGF without causing increased cell death or autophagy measured by increased LCA3/B. Our study provides a potential future strategy to deliver active Rap1a to the RPE in order to protect against both atrophic and neovascular AMD.
Conflict of interest statement
W.W.H. owns equity in AGTC and BionicSight LLC, is a paid consultant for AGTC and is a member of the AGTC scientific board. The other authors declare no any competing financial and/or non-financial interests in relation to the work presented here.
Figures





Similar articles
-
Retinal pigment epithelial cell expression of active Rap 1a by scAAV2 inhibits choroidal neovascularization.Mol Ther Methods Clin Dev. 2016 Aug 24;3:16056. doi: 10.1038/mtm.2016.56. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27606349 Free PMC article.
-
Rap1 GTPase activation and barrier enhancement in rpe inhibits choroidal neovascularization in vivo.PLoS One. 2013 Sep 10;8(9):e73070. doi: 10.1371/journal.pone.0073070. eCollection 2013. PLoS One. 2013. PMID: 24039860 Free PMC article.
-
Development of gene therapy for treatment of age-related macular degeneration.Acta Ophthalmol. 2014 Jul;92 Thesis3:1-38. doi: 10.1111/aos.12452. Acta Ophthalmol. 2014. PMID: 24953666
-
Suppression of Choroidal Neovascularization in Mice by Subretinal Delivery of Multigenic Lentiviral Vectors Encoding Anti-Angiogenic MicroRNAs.Hum Gene Ther Methods. 2017 Aug;28(4):222-233. doi: 10.1089/hgtb.2017.079. Hum Gene Ther Methods. 2017. PMID: 28817343
-
Regulation of signaling events involved in the pathophysiology of neovascular AMD.Mol Vis. 2016 Feb 27;22:189-202. eCollection 2016. Mol Vis. 2016. PMID: 27013848 Free PMC article. Review.
Cited by
-
CRISPR/Cas9 mediated specific ablation of vegfa in retinal pigment epithelium efficiently regresses choroidal neovascularization.Sci Rep. 2023 Mar 6;13(1):3715. doi: 10.1038/s41598-023-29014-z. Sci Rep. 2023. PMID: 36878916 Free PMC article.
-
Active Rap1-mediated inhibition of choroidal neovascularization requires interactions with IQGAP1 in choroidal endothelial cells.FASEB J. 2021 Jul;35(7):e21642. doi: 10.1096/fj.202100112R. FASEB J. 2021. PMID: 34166557 Free PMC article.
-
NF-κB activation in retinal microglia is involved in the inflammatory and neovascularization signaling in laser-induced choroidal neovascularization in mice.Exp Cell Res. 2021 Jun 1;403(1):112581. doi: 10.1016/j.yexcr.2021.112581. Epub 2021 Mar 31. Exp Cell Res. 2021. PMID: 33811906 Free PMC article.
-
AAV-Based Strategies for Treatment of Retinal and Choroidal Vascular Diseases: Advances in Age-Related Macular Degeneration and Diabetic Retinopathy Therapies.BioDrugs. 2024 Jan;38(1):73-93. doi: 10.1007/s40259-023-00629-y. Epub 2023 Oct 25. BioDrugs. 2024. PMID: 37878215 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

