Seconds-resolved pharmacokinetic measurements of the chemotherapeutic irinotecan in situ in the living body
- PMID: 31673321
- PMCID: PMC6788505
- DOI: 10.1039/c9sc01495k
Seconds-resolved pharmacokinetic measurements of the chemotherapeutic irinotecan in situ in the living body
Abstract
The ability to measure drugs in the body rapidly and in real time would advance both our understanding of pharmacokinetics and our ability to optimally dose and deliver pharmacological therapies. To this end, we are developing electrochemical aptamer-based (E-AB) sensors, a seconds-resolved platform technology that, as critical for performing measurements in vivo, is reagentless, reversible, and selective enough to work when placed directly in bodily fluids. Here we describe the development of an E-AB sensor against irinotecan, a member of the camptothecin family of cancer chemotherapeutics, and its adaptation to in vivo sensing. To achieve this we first re-engineered (via truncation) a previously reported DNA aptamer against the camptothecins to support high-gain E-AB signaling. We then co-deposited the modified aptamer with an unstructured, redox-reporter-modified DNA sequence whose output was independent of target concentration, rendering the sensor's signal gain a sufficiently strong function of square-wave frequency to support kinetic-differential-measurement drift correction. The resultant, 200 μm-diameter, 3 mm-long sensor achieves 20 s-resolved, multi-hour measurements of plasma irinotecan when emplaced in the jugular veins of live rats, thus providing an unprecedentedly high-precision view into the pharmacokinetics of this class of chemotherapeutics.
This journal is © The Royal Society of Chemistry 2019.
Figures

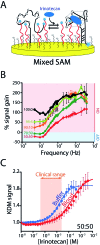
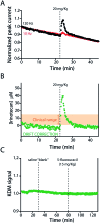


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

