Glucocorticoids preserve the t-tubular system in ventricular cardiomyocytes by upregulation of autophagic flux
- PMID: 31673803
- PMCID: PMC9380897
- DOI: 10.1007/s00395-019-0758-6
Glucocorticoids preserve the t-tubular system in ventricular cardiomyocytes by upregulation of autophagic flux
Abstract
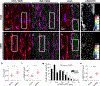
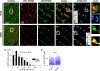
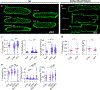
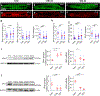
A major contributor to contractile dysfunction in heart failure is remodelling and loss of the cardiomyocyte transverse tubular system (t-system), but underlying mechanisms and signalling pathways remain elusive. It has been shown that dexamethasone promotes t-tubule development in stem cell-derived cardiomyocytes and that cardiomyocyte-specific glucocorticoid receptor (GR) knockout (GRKO) leads to heart failure. Here, we studied if the t-system is altered in GRKO hearts and if GR signalling is required for t-system preservation in adult cardiomyocytes. Confocal and 3D STED microscopy of myocardium from cardiomyocyte-specific GRKO mice revealed decreased t-system density and increased distances between ryanodine receptors (RyR) and L-type Ca2+ channels (LTCC). Because t-system remodelling and heart failure are intertwined, we investigated the underlying mechanisms in vitro. Ventricular cardiomyocytes from failing human and healthy adult rat hearts cultured in the absence of glucocorticoids (CTRL) showed distinctively lower t-system density than cells treated with dexamethasone (EC50 1.1 nM) or corticosterone. The GR antagonist mifepristone abrogated the effect of dexamethasone. Dexamethasone improved RyR-LTCC coupling and synchrony of intracellular Ca2+ release, but did not alter expression levels of t-system-associated proteins junctophilin-2 (JPH2), bridging integrator-1 (BIN1) or caveolin-3 (CAV3). Rather, dexamethasone upregulated LC3B and increased autophagic flux. The broad-spectrum protein kinase inhibitor staurosporine prevented dexamethasone-induced upregulation of autophagy and t-system preservation, and autophagy inhibitors bafilomycin A and chloroquine accelerated t-system loss. Conversely, induction of autophagy by rapamycin or amino acid starvation preserved the t-system. These findings suggest that GR signalling and autophagy are critically involved in t-system preservation and remodelling in the heart.
Keywords: Autophagy; Calcium signalling; Excitation–contraction coupling; Glucocorticoid receptor; Heart failure; Remodelling; Transverse tubular system.
Conflict of interest statement
Conflict of Interest
None declared.
Figures



References
-
- Bryant SM, Kong CHT, Watson JJ, Gadeberg HC, Roth DM, Patel HH, Cannell MB, James AF, Orchard CH (2018) Caveolin-3 KO disrupts t-tubule structure and decreases t-tubular ICa density in mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 315:H1101–H1111 doi:10.1152/ajpheart.00209.2018 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

