Effect of Abaloparatide vs Alendronate on Fracture Risk Reduction in Postmenopausal Women With Osteoporosis
- PMID: 31674644
- PMCID: PMC7112966
- DOI: 10.1210/clinem/dgz162
Effect of Abaloparatide vs Alendronate on Fracture Risk Reduction in Postmenopausal Women With Osteoporosis
Erratum in
-
ERRATUMERRATUM for "Effect of Abaloparatide vs Alendronate on Fracture Risk Reduction in Postmenopausal Women With Osteoporosis".J Clin Endocrinol Metab. 2020 Aug 1;105(8):e3053. doi: 10.1210/clinem/dgaa301. J Clin Endocrinol Metab. 2020. PMID: 32516411 Free PMC article. No abstract available.
Abstract
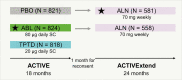
Context: The ACTIVE study demonstrated the antifracture efficacy of abaloparatide in postmenopausal women with osteoporosis. ACTIVExtend demonstrated sustained fracture risk reduction with alendronate in abaloparatide-treated participants from ACTIVE. A direct comparison of the efficacy of abaloparatide and antiresorptive therapies has not been performed.
Objective: The objective of this analysis is to compare the antifracture efficacy of abaloparatide in ACTIVE with that of alendronate in ACTIVExtend.
Design: In this post hoc analysis, the rate of new vertebral fractures for women in ACTIVExtend (N = 1139) was calculated based on baseline and endpoint radiographs for placebo or abaloparatide in ACTIVE and alendronate in ACTIVExtend. Vertebral fracture rates between abaloparatide and alendronate were compared in a Poisson regression model. Fracture rates for nonvertebral and clinical fractures were compared based on a Poisson model during 18 months of abaloparatide or placebo treatment in ACTIVE and 18 months of alendronate treatment in ACTIVExtend.
Results: The vertebral fracture rate was lower during abaloparatide treatment in ACTIVE (0.47 fractures/100 patient-years) than alendronate treatment in ACTIVExtend (1.66 fractures/100 patient-years) (relative risk reduction 71%; P = .027). Although the comparisons did not meet statistical significance, after switching from placebo (ACTIVE) to alendronate (ACTIVExtend), the rate of new vertebral fractures decreased from 2.49 to 1.66 fractures per 100 patient-years, and after switching from abaloparatide to alendronate from 0.47 to 0.19 fractures per 100 patient-years. The rates of nonvertebral fractures and clinical fractures were not significantly different.
Conclusion: Initial treatment with abaloparatide may result in greater vertebral fracture reduction compared with alendronate in postmenopausal women with osteoporosis.
Trial registration: ClinicalTrials.gov NCT01343004 NCT01657162.
Keywords: abaloparatide; alendronate; nonvertebral fractures; osteoporosis; vertebral fractures.
© Endocrine Society 2019.
Figures

Comment in
-
Osteoanabolics Versus Antiresorptives: Which First?J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgz238. doi: 10.1210/clinem/dgz238. J Clin Endocrinol Metab. 2020. PMID: 31782489 No abstract available.
References
-
- Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General. https://www.ncbi.nlm.nih.gov/books/NBK45513/. 2004. Accessed June 6, 2019. - PubMed
-
- Camacho PM, Petak SM, Binkley N, et al. . American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(Suppl 4):1–42. - PubMed
-
- Singer A, Exuzides A, Spangler L, et al. . Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clin Proc. 2015;90(1):53–62. - PubMed

