Cardiac ischemia-reperfusion injury induces ROS-dependent loss of PKA regulatory subunit RIα
- PMID: 31674811
- PMCID: PMC6962616
- DOI: 10.1152/ajpheart.00237.2019
Cardiac ischemia-reperfusion injury induces ROS-dependent loss of PKA regulatory subunit RIα
Abstract
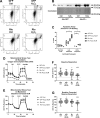
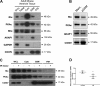
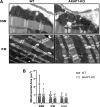
Type I PKA regulatory α-subunit (RIα; encoded by the Prkar1a gene) serves as the predominant inhibitor protein of the catalytic subunit of cAMP-dependent protein kinase (PKAc). However, recent evidence suggests that PKA signaling can be initiated by cAMP-independent events, especially within the context of cellular oxidative stress such as ischemia-reperfusion (I/R) injury. We determined whether RIα is actively involved in the regulation of PKA activity via reactive oxygen species (ROS)-dependent mechanisms during I/R stress in the heart. Induction of ex vivo global I/R injury in mouse hearts selectively downregulated RIα protein expression, whereas RII subunit expression appears to remain unaltered. Cardiac myocyte cell culture models were used to determine that oxidant stimulus (i.e., H2O2) alone is sufficient to induce RIα protein downregulation. Transient increase of RIα expression (via adenoviral overexpression) negatively affects cell survival and function upon oxidative stress as measured by increased induction of apoptosis and decreased mitochondrial respiration. Furthermore, analysis of mitochondrial subcellular fractions in heart tissue showed that PKA-associated proteins are enriched in subsarcolemmal mitochondria (SSM) fractions and that loss of RIα is most pronounced at SSM upon I/R injury. These data were supported via electron microscopy in A-kinase anchoring protein 1 (AKAP1)-knockout mice, where loss of AKAP1 expression leads to aberrant mitochondrial morphology manifested in SSM but not interfibrillar mitochondria. Thus, we conclude that modification of RIα via ROS-dependent mechanisms induced by I/R injury has the potential to sensitize PKA signaling in the cell without the direct use of the canonical cAMP-dependent activation pathway.NEW & NOTEWORTHY We uncovered a previously undescribed phenomenon involving oxidation-induced activation of PKA signaling in the progression of cardiac ischemia-reperfusion injury. Type I PKA regulatory subunit RIα, but not type II PKA regulatory subunits, is dynamically regulated by oxidative stress to trigger the activation of the catalytic subunit of PKA in cardiac myocytes. This effect may play a critical role in the regulation of subsarcolemmal mitochondria function upon the induction of ischemic injury in the heart.
Keywords: A-kinase anchoring protein 1; PKA regulatory subunit RIα; ischemia-reperfusion injury; mitochondria; oxidative stress.
Conflict of interest statement
H. H. Patel has equity as a founder in CavoGene LifeSciences Holdings, LLC.
Figures




Similar articles
-
Loss of PKA regulatory subunit 1α aggravates cardiomyocyte necrosis and myocardial ischemia/reperfusion injury.J Biol Chem. 2021 Jul;297(1):100850. doi: 10.1016/j.jbc.2021.100850. Epub 2021 Jun 1. J Biol Chem. 2021. PMID: 34087234 Free PMC article.
-
Essential Role of the RIα Subunit of cAMP-Dependent Protein Kinase in Regulating Cardiac Contractility and Heart Failure Development.Circulation. 2024 Dec 17;150(25):2031-2045. doi: 10.1161/CIRCULATIONAHA.124.068858. Epub 2024 Oct 2. Circulation. 2024. PMID: 39355927
-
Phosphorylation of protein kinase A (PKA) regulatory subunit RIα by protein kinase G (PKG) primes PKA for catalytic activity in cells.J Biol Chem. 2018 Mar 23;293(12):4411-4421. doi: 10.1074/jbc.M117.809988. Epub 2018 Jan 29. J Biol Chem. 2018. PMID: 29378851 Free PMC article.
-
Chemoprevention with protein kinase A RIalpha antisense in DMBA-mammary carcinogenesis.Ann N Y Acad Sci. 2005 Nov;1058:255-64. doi: 10.1196/annals.1359.038. Ann N Y Acad Sci. 2005. PMID: 16394142 Review.
-
Mitochondrial Kinase Signaling for Cardioprotection.Int J Mol Sci. 2024 Apr 19;25(8):4491. doi: 10.3390/ijms25084491. Int J Mol Sci. 2024. PMID: 38674076 Free PMC article. Review.
Cited by
-
From structure to the dynamic regulation of a molecular switch: A journey over 3 decades.J Biol Chem. 2021 Jan-Jun;296:100746. doi: 10.1016/j.jbc.2021.100746. Epub 2021 May 3. J Biol Chem. 2021. PMID: 33957122 Free PMC article. Review.
-
Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction.Cells. 2021 Apr 16;10(4):922. doi: 10.3390/cells10040922. Cells. 2021. PMID: 33923648 Free PMC article. Review.
-
Thermogenic Adipose Redox Mechanisms: Potential Targets for Metabolic Disease Therapies.Antioxidants (Basel). 2023 Jan 14;12(1):196. doi: 10.3390/antiox12010196. Antioxidants (Basel). 2023. PMID: 36671058 Free PMC article. Review.
-
Peptide Correlation Analysis (PeCorA) Reveals Differential Proteoform Regulation.J Proteome Res. 2021 Apr 2;20(4):1972-1980. doi: 10.1021/acs.jproteome.0c00602. Epub 2020 Dec 16. J Proteome Res. 2021. PMID: 33325715 Free PMC article.
-
Mitochondrial A-kinase anchoring proteins in cardiac ventricular myocytes.Physiol Rep. 2021 Sep;9(17):e15015. doi: 10.14814/phy2.15015. Physiol Rep. 2021. PMID: 34514737 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

