Efficacy of alemtuzumab over 6 years in relapsing-remitting multiple sclerosis patients who relapsed between courses 1 and 2: Post hoc analysis of the CARE-MS studies
- PMID: 31675266
- PMCID: PMC7604550
- DOI: 10.1177/1352458519881759
Efficacy of alemtuzumab over 6 years in relapsing-remitting multiple sclerosis patients who relapsed between courses 1 and 2: Post hoc analysis of the CARE-MS studies
Abstract
Background: Alemtuzumab is administered as two annual courses for relapsing-remitting multiple sclerosis (MS). Patients may relapse before completing the two-course regimen.
Objective: The objective was to evaluate 6-year outcomes in patients who relapsed between alemtuzumab Courses 1 and 2 (early relapsers).
Methods: Post hoc analysis of patients from the Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis (CARE-MS) studies who enrolled in the extension.
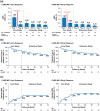
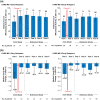
Results: Early relapsers (CARE-MS I: 15%; CARE-MS II: 24%) had more relapses in 1-2 years pre-alemtuzumab and higher mean baseline Expanded Disability Status Scale score than patients without relapse. Their annualized relapse rate declined from Year 1 (CARE-MS I: 1.3; CARE-MS II: 1.2) to Year 2 following Course 2 (0.3; 0.5) and remained low thereafter. Over 6 years, 60% remained free of 6-month confirmed disability worsening; 24% (CARE-MS I) and 34% (CARE-MS II) achieved 6-month confirmed disability improvement. During Year 6, 69% (CARE-MS I) and 68% (CARE-MS II) were free of magnetic resonance imaging (MRI) disease activity. Median percent yearly brain volume loss (Year 1: -0.67% (CARE-MS I); -0.47% (CARE-MS II)) declined after Course 2 (Year 6: -0.24%; -0.13%).
Conclusion: Early relapsers' outcomes improved after completing the second alemtuzumab course. These findings support administering the approved two-course regimen to maximize clinical benefit.
Clinicaltrials.gov registration numbers: CARE-MS I, II, extension: NCT00530348, NCT00548405, NCT00930553.
Keywords: Alemtuzumab; disability; disease-modifying therapy; efficacy; magnetic resonance imaging (MRI); relapsing–remitting multiple sclerosis (MS).
Conflict of interest statement
Figures


References
-
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1819–1828. - PubMed
-
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1829–1839. - PubMed

