Treatment beyond four cycles of first line Platinum and Etoposide chemotherapy in real-life patients with stage IV Small Cell Lung Cancer: a retrospective study of the Merseyside and Cheshire Cancer network
- PMID: 31675940
- PMCID: PMC6823940
- DOI: 10.1186/s12890-019-0948-x
Treatment beyond four cycles of first line Platinum and Etoposide chemotherapy in real-life patients with stage IV Small Cell Lung Cancer: a retrospective study of the Merseyside and Cheshire Cancer network
Abstract
Background: Dose intensity and dose density of first line Platinum and Etoposide (PE) do not influence Overall Survival (OS) of Small Cell Lung Cancer (SCLC) patients. The effect of treatment length, however, remains unclear. Current guidelines recommend treating beyond 4 cycles -up to 6-, in patients that respond to and tolerate systemic treatment. This has led to variable practice both in clinical practice and clinical research. Here we aimed at quantifying the possible clinical benefit of the extended regimen in our real-life patients treated with PE doublet.
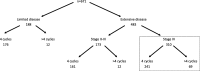
Methods: Of all patients with SCLC treated in our network with non-concurrent first line PE chemotherapy between 2008 and 2015, we identified and described patients that received 4 cycles (4c) or more (> 4c), and analysed patients with stage IV disease.
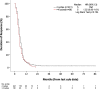
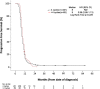
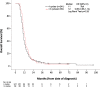
Results: Two hundred forty-one patients with stage IV had 4c and 69 had > 4c. The latter were more likely to have sequential thoracic radiotherapy, which suggested a lower metastatic burden. Nevertheless, there were no statistically significant differences when comparing clinical outcomes. The median Duration of Response (DoR; time from last chemotherapy cycle to progression) was 5 months in both groups (HR 1.22; 95% CI 0.93-1.61). Median Progression Free Survival (PFS; time from diagnosis to radiological progression) was 8 months (4c) versus 9 months (> 4c) (HR 0.86; 95% CI 0.66-1.13) and median OS was 11 versus 12 months (HR 0.86, 95% CI 0.66-1.14).
Conclusion: Our results highlight a lack of clinical benefit by extending first line PE treatment in stage IV disease, and support limiting treatment to 4 cycles until superiority of a longer regimen is identified in a randomised study.
Keywords: Antineoplastic combined chemotherapy protocols; Drug therapy; Lung neoplasm; Observational study; Small cell lung carcinoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Foster NR, Qi Y, Shi Q, Krook JE, Kugler JW, Jett JR, et al. Tumor response and progression-free survival as potential surrogate endpoints for overall survival in extensive stage small-cell lung cancer: findings on the basis of north central Cancer treatment group trials. Cancer. 2011;117(6):1262–1271. doi: 10.1002/cncr.25526. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

