Magnitude and causes of first-line antiretroviral therapy regimen changes among HIV patients in Ethiopia: a systematic review and meta-analysis
- PMID: 31675986
- PMCID: PMC6824137
- DOI: 10.1186/s40360-019-0361-3
Magnitude and causes of first-line antiretroviral therapy regimen changes among HIV patients in Ethiopia: a systematic review and meta-analysis
Abstract
Background: Antiretroviral therapy (ART) has markedly decreased the morbidity and mortality due to HIV/AIDS. ART regimen change is a major challenge for the sustainability of human immunodeficiency virus (HIV) treatment program. This is found to be a major concern among HIV/AIDS patients in a resource-limited setting, where treatment options are limited.
Objectives: The aim of this review is to generate the best available evidence regarding the magnitude of first-line antiretroviral therapy regimen change and the causes for regimen change among HIV patients on ART in Ethiopia.
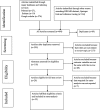
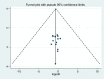
Methods: The reviewed studies were accessed through electronic web-based search strategy from PubMed Medline, EMBASE, Hinari, Springer link and Google Scholar. Data were extracted using Microsoft Excel and exported to Stata software version 13 for analyses. The overall pooled estimation of outcomes was calculated using a random-effect model of DerSimonian-Laird method at 95% confidence level. Heterogeneity of studies was determined using I2 statistics. For the magnitude of regimen change, the presence of publication bias was evaluated using the Begg's and Egger's tests. The protocol of this systematic review and meta-analysis was registered in the Prospero database with reference number ID: CRD42018099742. The published methodology is available from: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=99742 .
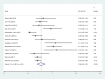
Results: A total of 22 studies published between the years 2012 and 2018 were included. Out of 22 articles, 14 articles reported the magnitude of regimen change and consisted of 13,668 HIV patients. The estimated national pooled magnitude of regimen change was 37% (95% CI: 34, 44%; Range: 15.1-63.8%) with degree of heterogeneity (I2), 98.7%; p-value < 0.001. Seventeen articles were used to identify the causes for first-line antiretroviral therapy regimen change. The major causes identified were toxicity, 58% (95% CI: 46, 69%; Range: 14.4-88.5%); TB co-morbidity, 12% (95% CI: 8, 16%; Range: 0.8-31.7%); treatment failure, 7% (95% CI: 5, 9%; Range: 0.4-24.4%); and pregnancy, 5% (95% CI: 4, 7%; Range: 0.6-11.9%).
Conclusions: The original first-line regimen was changed in one-third of HIV patients on ART in Ethiopia. Toxicity of the drugs, TB co-morbidity, treatment failure, and pregnancy were the main causes for the change of the first-line regimen among HIV patients on antiretroviral therapy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- UNAIDS. Global AIDSUpdate 2016. Geneva, Switzerland. Available from: https://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. Accessed 15 Aug 2018.
-
- Joint United Nations Programme on HIV/AIDS. Fact sheet—Latest statistics on the status of the AIDS epidemic. 2017. Available from: https://www.unaids.org/en/resources/fact-sheet. Accessed 20 Aug 2018.
-
- CSA.Ethiopia Demographic and Health Survey. HIV prevalence report. Central Statistical Authority. 2016;2016.
-
- Yared M, Tibebu S, Emmart P. Equity and access to ART in Ethiopia. Washington, DC: Future Group, Health Policy Initiative. Task Order I; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

