Multisensory Integration and the Society for Neuroscience: Then and Now
- PMID: 31676599
- PMCID: PMC6939490
- DOI: 10.1523/JNEUROSCI.0737-19.2019
Multisensory Integration and the Society for Neuroscience: Then and Now
Abstract
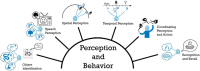
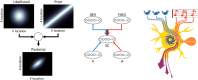
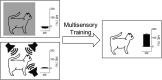
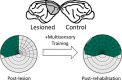
The operation of our multiple and distinct sensory systems has long captured the interest of researchers from multiple disciplines. When the Society was founded 50 years ago to bring neuroscience research under a common banner, sensory research was largely divided along modality-specific lines. At the time, there were only a few physiological and anatomical observations of the multisensory interactions that powerfully influence our everyday perception. Since then, the neuroscientific study of multisensory integration has increased exponentially in both volume and diversity. From initial studies identifying the overlapping receptive fields of multisensory neurons, to subsequent studies of the spatial and temporal principles that govern the integration of multiple sensory cues, our understanding of this phenomenon at the single-neuron level has expanded to include a variety of dimensions. We now can appreciate how multisensory integration can alter patterns of neural activity in time, and even coordinate activity among populations of neurons across different brain areas. There is now a growing battery of sophisticated empirical and computational techniques that are being used to study this process in a number of models. These advancements have not only enhanced our understanding of this remarkable process in the normal adult brain, but also its underlying circuitry, requirements for development, susceptibility to malfunction, and how its principles may be used to mitigate malfunction.
Copyright © 2020 the authors.
Figures




References
-
- Bremner AJ, Lewkowicz DJ, Spence C (eds) (2012) Multisensory development, Ed 1 Oxford: Oxford UP.
-
- Bruno N, Pavani F (2018) Perception: a multisensory perspective. Oxford: Oxford UP.
-
- Calvert G, Spence C, Stein BE (eds) (2004) Handbook of multisensory processes. Cambridge, MA: Massachusetts Institute of Technology.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
