Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction
- PMID: 31676603
- PMCID: PMC6891062
- DOI: 10.1523/JNEUROSCI.1142-19.2019
Disrupted Blood-Retina Lysophosphatidylcholine Transport Impairs Photoreceptor Health But Not Visual Signal Transduction
Abstract
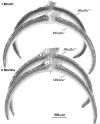
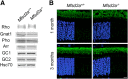
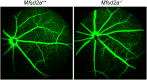
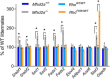
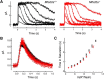
Retinal photoreceptor cells contain the highest concentration of docosahexaenoic acid (DHA) in our bodies, and it has been long assumed that this is critical for supporting normal vision. Indeed, early studies using DHA dietary restriction documented reduced light sensitivity by DHA-deprived retinas. Recently, it has been demonstrated that a major route of DHA entry in the retina is the delivery across the blood-retina barrier by the sodium-dependent lipid transporter, Mfsd2a. This discovery opened a unique opportunity to analyze photoreceptor health and function in DHA-deprived retinas using the Mfsd2a knock-out mouse as animal model. Our lipidome analyses of Mfsd2a-/- retinas and outer segment membranes corroborated the previously reported decrease in the fraction of DHA-containing phospholipids and a compensatory increase in phospholipids containing arachidonic acid. We also revealed an increase in the retinal content of monounsaturated fatty acids and a reduction in very long chain fatty acids. These changes could be explained by a combination of reduced DHA supply to the retina and a concomitant upregulation of several fatty acid desaturases controlled by sterol regulatory element-binding transcription factors, which are upregulated in Mfsd2a-/- retinas. Mfsd2a-/- retinas undergo slow progressive degeneration, with ∼30% of photoreceptor cells lost by the age of 6 months. Despite this pathology, the ultrastructure Mfsd2a-/- photoreceptors and their ability to produce light responses were essentially normal. These data demonstrate that, whereas maintaining the lysophosphatidylcholine route of DHA supply to the retina is essential for long-term photoreceptor survival, it is not important for supporting normal phototransduction.SIGNIFICANCE STATEMENT Phospholipids containing docosahexaenoic acid (DHA) are greatly enriched in the nervous system, with the highest concentration found in the light-sensitive membranes of photoreceptor cells. In this study, we analyzed the consequences of impaired DHA transport across the blood-retina barrier. We have found that, in addition to a predictable reduction in the DHA level, the affected retinas undergo a complex, transcriptionally-driven rebuilding of their membrane lipidome in a pattern preserving the overall saturation/desaturation balance of retinal phospholipids. Remarkably, these changes do not affect the ability of photoreceptors to produce responses to light but are detrimental for the long-term survival of these cells.
Keywords: DHA; Mfsd2a; phospholipids; photoreceptor; retina; retinal degeneration.
Copyright © 2019 the authors.
Figures






References
-
- Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH (2015) A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47:814–817. 10.1038/ng.3313 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
