Hyperglycemia-induced inflamm-aging accelerates gingival senescence via NLRC4 phosphorylation
- PMID: 31676687
- PMCID: PMC6901307
- DOI: 10.1074/jbc.RA119.010648
Hyperglycemia-induced inflamm-aging accelerates gingival senescence via NLRC4 phosphorylation
Abstract
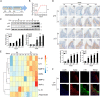
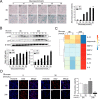
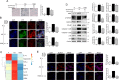
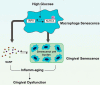
Inflamm-aging was recently affiliated with the progression of diabetic complications. Local cellular senescence together with senescence-associated secretory phenotype (SASP) are the main contributors to inflamm-aging. However, little is known about their involvement in diabetic periodontitis. Gingiva is the first line of host defense in the periodontium, and macrophages are key SASP-carrying cells. Here, we explored the molecular mechanism by which hyperglycemia drives the inflamm-aging in the gingival tissue of diabetic mice and macrophages. We demonstrated that hyperglycemia increased the infiltrated macrophage senescence in gingival tissue of diabetic mice. Simultaneously, hyperglycemia elevated the local burden of senescent cells in gingival tissue and induced the serum secretion of SASP factors in vivo Moreover, in vitro, high glucose induced macrophage senescence and SASP factors secretion through phosphorylation of NLRC4, which further stimulated the NF-κB/Caspase-1 cascade via an IRF8-dependent pathway. Deletion of NLRC4 or IRF8 abolished hyperglycemia-induced cellular senescence and SASP in macrophages. In addition, we found that treatment with metformin inhibited NLRC4 phosphorylation and remarkably decreased cellular senescence and SASP in the context of hyperglycemia. Our data demonstrated that hyperglycemia induces the development of inflamm-aging in gingival tissue and suggested that NLRC4 is a potential target for treatment of diabetes-associated complications.
Keywords: NLRC4; SASP; aging; cellular senescence; diabetes; gingiva; hyperglycemia; inflamm-aging; inflammasome; inflammation.
© 2019 Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging.J Clin Periodontol. 2021 Oct;48(10):1379-1392. doi: 10.1111/jcpe.13517. Epub 2021 Aug 18. J Clin Periodontol. 2021. PMID: 34219262
-
Hyperglycemia accelerates inflammaging in the gingival epithelium through inflammasomes activation.J Periodontal Res. 2021 Aug;56(4):667-678. doi: 10.1111/jre.12863. Epub 2021 Mar 2. J Periodontal Res. 2021. PMID: 33650689
-
Sustained exposure to high glucose induces differential expression of cellular senescence markers in murine macrophages but impairs immunosurveillance response to senescent cells secretome.Biogerontology. 2024 Aug;25(4):627-647. doi: 10.1007/s10522-024-10092-z. Epub 2024 Jan 19. Biogerontology. 2024. PMID: 38240923
-
MicroRNAs linking inflamm-aging, cellular senescence and cancer.Ageing Res Rev. 2013 Sep;12(4):1056-68. doi: 10.1016/j.arr.2013.05.001. Epub 2013 May 17. Ageing Res Rev. 2013. PMID: 23688930 Review.
-
DNA damage response (DDR) and senescence: shuttled inflamma-miRNAs on the stage of inflamm-aging.Oncotarget. 2015 Nov 3;6(34):35509-21. doi: 10.18632/oncotarget.5899. Oncotarget. 2015. PMID: 26431329 Free PMC article. Review.
Cited by
-
Erythritol Can Inhibit the Expression of Senescence Molecules in Mouse Gingival Tissues and Human Gingival Fibroblasts.Nutrients. 2023 Sep 19;15(18):4050. doi: 10.3390/nu15184050. Nutrients. 2023. PMID: 37764833 Free PMC article.
-
The Anti-Aging Mechanism of Metformin: From Molecular Insights to Clinical Applications.Molecules. 2025 Feb 10;30(4):816. doi: 10.3390/molecules30040816. Molecules. 2025. PMID: 40005128 Free PMC article. Review.
-
Pexidartinib Inhibits Macrophage Senescence Through Glycolysis in Periodontitis Microenvironment.Int Dent J. 2025 Aug;75(4):100843. doi: 10.1016/j.identj.2025.100843. Epub 2025 Jun 2. Int Dent J. 2025. PMID: 40460514 Free PMC article.
-
Role of inflammasomes in diabetes mellitus: mechanisms, complications, and therapeutic potential.Mol Biol Rep. 2025 Jun 21;52(1):621. doi: 10.1007/s11033-025-10719-5. Mol Biol Rep. 2025. PMID: 40544214 Review.
-
Editorial: Cellular Mechanisms of Aging and Longevity in Oral Health and Disease.Front Oral Health. 2022 Jul 12;3:971191. doi: 10.3389/froh.2022.971191. eCollection 2022. Front Oral Health. 2022. PMID: 35903612 Free PMC article. No abstract available.
References
-
- Demmer R. T., Holtfreter B., Desvarieux M., Jacobs D. R. Jr., Kerner W., Nauck M., Völzke H., and Kocher T. (2012) The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the Study of Health in Pomerania (SHIP). Diabetes Care 35, 2036–2042 10.2337/dc11-2453 - DOI - PMC - PubMed
-
- Waaijer M. E., Parish W. E., Strongitharm B. H., van Heemst D., Slagboom P. E., de Craen A. J., Sedivy J. M., Westendorp R. G., Gunn D. A., and Maier A. B. (2012) The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 11, 722–725 10.1111/j.1474-9726.2012.00837.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

