Residues and residue pairs of evolutionary importance differentially direct signaling bias of D2 dopamine receptors
- PMID: 31676688
- PMCID: PMC6916503
- DOI: 10.1074/jbc.RA119.008068
Residues and residue pairs of evolutionary importance differentially direct signaling bias of D2 dopamine receptors
Abstract
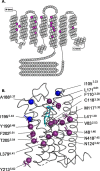
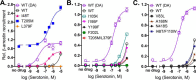
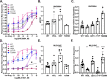
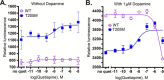
The D2 dopamine receptor and the serotonin 5-hydroxytryptamine 2A receptor (5-HT2A) are closely-related G-protein-coupled receptors (GPCRs) from the class A bioamine subfamily. Despite structural similarity, they respond to distinct ligands through distinct downstream pathways, whose dysregulation is linked to depression, bipolar disorder, addiction, and psychosis. They are important drug targets, and it is important to understand how their bias toward G-protein versus β-arrestin signaling pathways is regulated. Previously, evolution-based computational approaches, difference Evolutionary Trace and Evolutionary Trace-Mutual information (ET-Mip), revealed residues and residue pairs that, when switched in the D2 receptor to the corresponding residues from 5-HT2A, altered ligand potency and G-protein activation efficiency. We have tested these residue swaps for their ability to trigger recruitment of β-arrestin2 in response to dopamine or serotonin. The results reveal that the selected residues modulate agonist potency, maximal efficacy, and constitutive activity of β-arrestin2 recruitment. Whereas dopamine potency for most variants was similar to that for WT and lower than for G-protein activation, potency in β-arrestin2 recruitment for N124H3.42 was more than 5-fold higher. T205M5.54 displayed high constitutive activity, enhanced dopamine potency, and enhanced efficacy in β-arrestin2 recruitment relative to WT, and L379F6.41 was virtually inactive. These striking differences from WT activity were largely reversed by a compensating mutation (T205M5.54/L379F6.41) at residues previously identified by ET-Mip as functionally coupled. The observation that the signs and relative magnitudes of the effects of mutations in several cases are at odds with their effects on G-protein activation suggests that they also modulate signaling bias.
Keywords: G protein; G-protein–coupled receptor (GPCR); allosteric regulation; arrestin; cell signaling; dopamine; dopamine receptor.
© 2019 Terrón-Díaz et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Deslauriers J., Desmarais C., Sarret P., and Grignon S. (2013) α-Lipoic acid interaction with dopamine D2 receptor-dependent activation of the Akt/GSK-3β signaling pathway induced by antipsychotics: potential relevance for the treatment of schizophrenia. J. Mol. Neurosci. 50, 134–145 10.1007/s12031-012-9884-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

