Neratinib protects pancreatic beta cells in diabetes
- PMID: 31676778
- PMCID: PMC6825211
- DOI: 10.1038/s41467-019-12880-5
Neratinib protects pancreatic beta cells in diabetes
Abstract
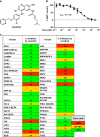
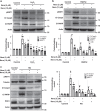
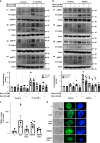
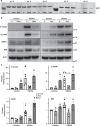
The loss of functional insulin-producing β-cells is a hallmark of diabetes. Mammalian sterile 20-like kinase 1 (MST1) is a key regulator of pancreatic β-cell death and dysfunction; its deficiency restores functional β-cells and normoglycemia. The identification of MST1 inhibitors represents a promising approach for a β-cell-protective diabetes therapy. Here, we identify neratinib, an FDA-approved drug targeting HER2/EGFR dual kinases, as a potent MST1 inhibitor, which improves β-cell survival under multiple diabetogenic conditions in human islets and INS-1E cells. In a pre-clinical study, neratinib attenuates hyperglycemia and improves β-cell function, survival and β-cell mass in type 1 (streptozotocin) and type 2 (obese Leprdb/db) diabetic mouse models. In summary, neratinib is a previously unrecognized inhibitor of MST1 and represents a potential β-cell-protective drug with proof-of-concept in vitro in human islets and in vivo in rodent models of both type 1 and type 2 diabetes.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

