Herpes Zoster Risk in Immunocompromised Adults in the United States: A Systematic Review
- PMID: 31677266
- PMCID: PMC7195255
- DOI: 10.1093/cid/ciz1090
Herpes Zoster Risk in Immunocompromised Adults in the United States: A Systematic Review
Abstract
Background: The primary reported risk factors for herpes zoster (HZ) include increasing age and immunodeficiency, yet estimates of HZ risk by immunocompromising condition have not been well characterized. We undertook a systematic literature review to estimate the HZ risk in immunocompromised patients.
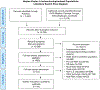
Methods: We systematically reviewed studies that examined the risk of HZ and associated complications in adult patients with hematopoietic cell transplants (HCT), cancer, human immunodeficiency virus (HIV), and solid organ transplant (SOT). We identified studies in PubMed, Embase, Medline, Cochrane, Scopus, and clinicaltrials.gov that presented original data from the United States and were published after 1992. We assessed the risk of bias with Cochrane or Grading of Recommendations Assessment, Development, and Evaluation methods.
Results: We identified and screened 3765 records and synthesized 34 studies with low or moderate risks of bias. Most studies that were included (32/34) reported at least 1 estimate of the HZ cumulative incidence (range, 0-41%). There were 12 studies that reported HZ incidences that varied widely within and between immunocompromised populations. Incidence estimates ranged from 9 to 92 HZ cases/1000 patient-years and were highest in HCT, followed by hematologic malignancies, SOT, and solid tumor malignancies, and were lowest in people living with HIV. Among 17 HCT studies, the absence of or use of antiviral prophylaxis at <1 year post-transplant was associated with a higher HZ incidence.
Conclusions: HZ was common among all immunocompromised populations studied, exceeding the expected HZ incidence among immunocompetent adults aged ≥60 years. Better evidence of the incidence of HZ complications and their severity in immunocompromised populations is needed to inform economic and HZ vaccine policies.
Keywords: RZV; VZV; herpes zoster; immunocompromised; postherpetic neuralgia.
Published by Oxford University Press for the Infectious Diseases Society of America 2019.
Conflict of interest statement
Potential Conflicts of Interest:
S.A.P. has received research funding from Global Life Technologies Corp, and has participated in clinical trials with Chimerix, Inc. All other authors: no reported conflicts of interest.
Figures


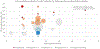
References
-
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic proceedings 2007; 82(11): 1341–9. - PubMed
-
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2011; 52(3): 332–40. - PubMed
-
- Prosser LA, Harpaz R, Rose AM, et al. A Cost-Effectiveness Analysis of Vaccination for Prevention of Herpes Zoster and Related Complications: Input for National Recommendations. Ann Intern Med 2019; 170(6): 380–8. - PubMed
-
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6(6): 356–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

