Polyfunctional, Proinflammatory, Tissue-Resident Memory Phenotype and Function of Synovial Interleukin-17A+CD8+ T Cells in Psoriatic Arthritis
- PMID: 31677365
- PMCID: PMC7065207
- DOI: 10.1002/art.41156
Polyfunctional, Proinflammatory, Tissue-Resident Memory Phenotype and Function of Synovial Interleukin-17A+CD8+ T Cells in Psoriatic Arthritis
Abstract
Objective: Genetic associations imply a role for CD8+ T cells and the interleukin-23 (IL-23)/IL-17 axis in psoriatic arthritis (PsA) and other spondyloarthritides (SpA). IL-17A+CD8+ (Tc17) T cells are enriched in the synovial fluid (SF) of patients with PsA, and IL-17A blockade is clinically efficacious in PsA/SpA. This study was undertaken to determine the immunophenotype, molecular profile, and function of synovial Tc17 cells in order to elucidate their role in PsA/SpA pathogenesis.
Methods: Peripheral blood (PB) and SF mononuclear cells were isolated from patients with PsA or other types of SpA. Cells were phenotypically, transcriptionally, and functionally analyzed by flow cytometry (n = 6-18), T cell receptor β (TCRβ) sequencing (n = 3), RNA-Seq (n = 3), quantitative reverse transcriptase-polymerase chain reaction (n = 4), and Luminex or enzyme-linked immunosorbent assay (n = 4-16).
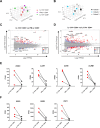
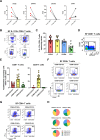
Results: IL-17A+CD8+ T cells were predominantly TCRαβ+ and their frequencies were increased in the SF versus the PB of patients with established PsA (P < 0.0001) or other SpA (P = 0.0009). TCRβ sequencing showed that these cells were polyclonal in PsA (median clonality 0.08), while RNA-Seq and deep immunophenotyping revealed that PsA synovial Tc17 cells had hallmarks of Th17 cells (RORC/IL23R/CCR6/CD161) and Tc1 cells (granzyme A/B). Synovial Tc17 cells showed a strong tissue-resident memory T (Trm) cell signature and secreted a range of proinflammatory cytokines. We identified CXCR6 as a marker for synovial Tc17 cells, and increased levels of CXCR6 ligand CXCL16 in PsA SF (P = 0.0005), which may contribute to their retention in the joint.
Conclusion: Our results identify synovial Tc17 cells as a polyclonal subset of Trm cells characterized by polyfunctional, proinflammatory mediator production and CXCR6 expression. The molecular signature and functional profiling of these cells may help explain how Tc17 cells can contribute to synovial inflammation and disease persistence in PsA and possibly other types of SpA.
© 2019, The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures




References
-
- Taams LS, Steel KJ, Srenathan U, Burns LA, Kirkham BW. IL‐17 in the immunopathogenesis of spondyloarthritis. Nat Rev Rheumatol 2018;14:453–66. - PubMed
-
- Gravallese EM, Schett G. Effects of the IL‐23‐IL‐17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol 2018;14:631–40. - PubMed
-
- Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin‐17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. - PubMed
-
- Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin‐17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

