(-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation
- PMID: 31677553
- PMCID: PMC6920094
- DOI: 10.1016/j.redox.2019.101360
(-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation
Abstract
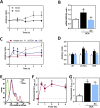
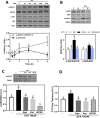
Secondary bile acids promote gastrointestinal (GI) tract permeabilization both in vivo and in vitro. Consumption of high fat diets increases bile acid levels in the GI tract which can contribute to intestinal permeabilization and consequent local and systemic inflammation. This work investigated the mechanisms involved in bile acid (deoxycholic acid (DCA))-induced intestinal epithelial cell monolayer permeabilization and the preventive capacity of (-)-epicatechin (EC). While EC prevented high fat diet-induced intestinal permeabilization in mice, it did not mitigate the associated increase in fecal/cecal total and individual bile acids. In vitro, using differentiated Caco-2 cells as a model of epithelial barrier, EC and other NADPH oxidase inhibitors (VAS-2870 and apocynin) mitigated DCA-induced Caco-2 monolayer permeabilization. While EC inhibited DCA-mediated increase in cell oxidants, it did not prevent DCA-induced mitochondrial oxidant production. Prevention of DCA-induced ERK1/2 activation with EC, VAS-2870, apocynin and the MEK inhibitor U0126, also prevented monolayer permeabilization, stressing the key involvement of ERK1/2 in this process and its redox regulation. Downstream, DCA promoted myosin light chain (MLC) phosphorylation which was related to MLC phosphatase (MLCP) inhibition by ERK1/2. DCA also decreased the levels of the tight junction proteins ZO-1 and occludin, which can be related to MMP-2 activation and consequent ZO-1 and occludin degradation. Both events were prevented by EC, NADPH oxidase and ERK1/2 inhibitors. Thus, DCA-induced Caco-2 monolayer permeabilization occurs mainly secondary to a redox-regulated ERK1/2 activation and downstream disruption of TJ structure and dynamic. EC's capacity to mitigate in vivo the gastrointestinal permeabilization caused by consumption of high-fat diets can be in part related to its capacity to inhibit bile-induced NADPH oxidase and ERK1/2 activation.
Keywords: Bile acids; Deoxycholic acid; Epicatechin; High fat; Intestinal permeability.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None of the authors has any activity that represents a conflict of interest with this work.
Figures





References
-
- Gadaleta R.M., Garcia-Irigoyen O., Moschetta A. Bile acids and colon cancer: is FXR the solution of the conundrum? Mol. Asp. Med. 2017:66–74. - PubMed
-
- Damms-Machado A., Louis S., Schnitzer A., Volynets V., Rings A., Basrai M., Bischoff S.C. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am. J. Clin. Nutr. 2017;1:127–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

